Donna Rivera
@drdonnarivera
Associate Director of Pharmacoepidemiology @FDAOncology. Passionate about optimizing #RWD for good 🌎 Opinions are my own. #RWE #EpiTwitter
ID: 1356086920187686918
01-02-2021 03:48:39
151 Tweet
288 Followers
268 Following

Out of an incredible collaborative effort, COVID-19 and Cancer Consortium (CCC19) Registry generates #RWD to better understand #COVID19 for patients with cancer. ➡️And recently, this paper was featured as a most cited 2020-2021 publication in Cancer Discovery! Team Effort! 👏🏻 Solange Peters @hemoncwarner



A great way to start the day➡️ Warm welcome from National Organization for Rare Disorders (NORD) community at #NORDSummit! Joining U.S. FDA colleagues to collaboratively discuss evidence generation using #RWD across centers! FDA Biologics FDA Medical Devices FDA Drug Information FDA Oncology #RareDisease


Thrilled to see colleagues Friends of Cancer Research Meeting and hear U.S. FDA and FDA Oncology leaders Dr. Robert M. Califf Rick Pazdur discuss the importance of accelerated approvals, generalizability, and Project Pragmáctica to enhance trial efficiencies and patient centricity. #FriendsAM22


Interesting panel discussion on approaches to including Patient-Reported Outcomes in Dose Optimization ➡️ explaining the"why" of data collection and emphasizing equity with Vishal Bhatnagar, MD and Paul Kluetz. FDA Oncology #FriendsAM22 #ProjectOptimus #OCEPFDD


Great to hear the emphasis on patient-centric across agency #collaboration toward advancing #CancerResearch and #clinicaltrials together from Dr. Kimryn Rathmell Dr. Monica Bertagnolli in conversation with Friends of Cancer Research Founder Ellen Sigal! FDA Oncology #FriendsAM22 #CancerMoonshot


Great discussion with Ellen Sigal this afternoon at the Friends of Cancer Research Annual Meeting! Partnerships across the #CancerResearch community are an instrumental element of progress. #FriendsAM22 #WhatFriendsDoes


First and foremost, our partnerships must focus on what people with cancer and #CancerCare providers need. Together, we will work to #EndCancerAsWeKnowIt. Friends of Cancer Research White House Office of Science & Technology Policy ARPA-H U.S. FDA FDA Oncology NIH National Cancer Institute

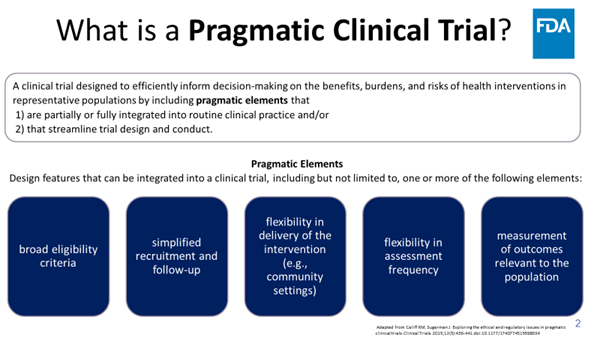
.FDA Oncology's Harpreet Singh, MD and Donna Rivera describe the “new normal”—and the future—in cancer pragmatic trials. cancerletter.com/conversation-w…

TODAY: Join for the FDA 8th Annual Clinical Outcome Assessment in Cancer Clinical Trials (COA-CCT) Workshop with opening remarks by OCE Deputy Director Paul Kluetz #OCEOutcomes23 Event details: fda.gov/news-events/fd…


The FDA Oncology Center of Excellence established a collaboration with Foundation for FDA to enhance facilitation of effective communication & characterization of data source(s) and study design in early #RWD study proposals for #oncology . FDA Oncology #FDAVoices #OCERWE #RxEpi #RWE




New #AI initiative ASCO: Check out the first meeting of the #AI Community of Practice (#CoP) this Friday #ASCO24! We're gathering a community of clinicians, researchers, implementers, & educators looking to integrate AI responsibly in #oncology practice. conferences.asco.org/am/communities…



FDA’s Oncology Center of Excellence Launches Project 5 in 5, a Crowdsourcing Initiative - via The ASCO Post ▶️ ascopost.com/issues/june-10… By Steven C. Cunningham Donna Rivera Kirsten B. Goldberg Rick Pazdur @KellyNorsworth2 Jennifer Gao #OCEProject5in5 #PragmaticTrials