Huakui Liu
@huakuiliu
Shanghai Institute of Organic Chemistry
ID: 761702090859175938
05-08-2016 23:15:05
96 Tweet
53 Followers
92 Following

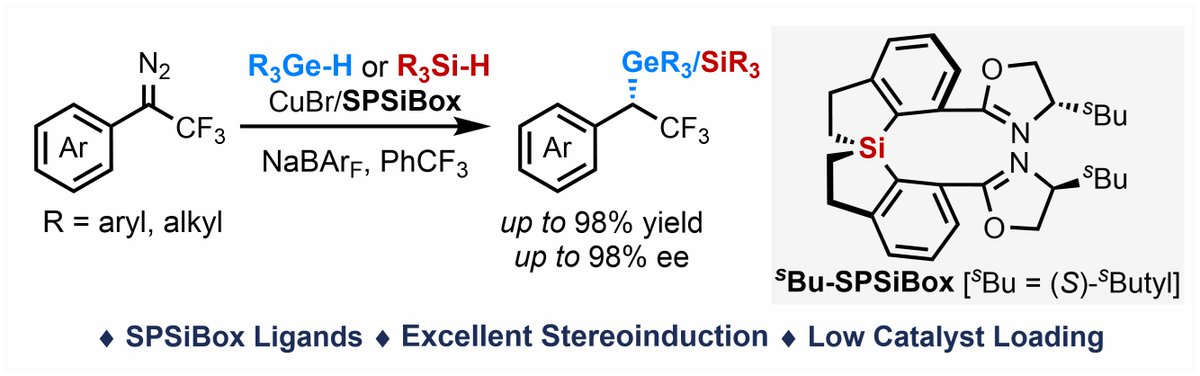
Fantastic first work by former group member Youzhi Xu-徐尤智 at Henan U!👇 It is hard enough to make a hetero[3]catenane. They did it in 35% yield via "ring-in-ring synthesis" from two commercial macrocycles😮!!! We were very happy to help with MS-MS studies. x.com/xu_youzhi/stat…
![Delius Lab 🇪🇺🇺🇦 (@mvdelius) on Twitter photo Fantastic first work by former group member <a href="/xu_youzhi/">Youzhi Xu-徐尤智</a> at Henan U!👇 It is hard enough to make a hetero[3]catenane. They did it in 35% yield via "ring-in-ring synthesis" from two commercial macrocycles😮!!! We were very happy to help with MS-MS studies.
x.com/xu_youzhi/stat… Fantastic first work by former group member <a href="/xu_youzhi/">Youzhi Xu-徐尤智</a> at Henan U!👇 It is hard enough to make a hetero[3]catenane. They did it in 35% yield via "ring-in-ring synthesis" from two commercial macrocycles😮!!! We were very happy to help with MS-MS studies.
x.com/xu_youzhi/stat…](https://pbs.twimg.com/media/GaFkaClXwAAfM65.png)

Just in time for Halloween💀☠️ skeletal editing of rotaxanes! Maxime Gauthier, Jess & Dan use Mark Levin’s N-deletion reagent to remove template site of dibenzylammonium rotaxanes enabling synthesis of otherwise inaccessible structures.Out now in J. Am. Chem. Soc. pubs.acs.org/doi/10.1021/ja…




Glad to see our flexible Zn4L4 cage published in J. Am. Chem. Soc. . This project was a collaborative effort across three institutions, with solid support from colleagues at #Sichuan Uinversity, Clever Clever Lab and Nitschke Cambridge Chemistry groups.

Finally out in Nature Communications With a bulky acac ligand, we have realized the Ni(II)-catalyzed dicarbofunctionalization of unactivated alkenes with a diverse range of native functional groups as the directing group. Check out: rdcu.be/d1RZD



If you haven’t read Dave Leigh group piece on molecular editing of rotaxanes yet (surl.li/nyivhc), perhaps our highlight (surl.li/cbtjhf ) in ChemPlusChem - written with Jędrzej Perdek and Rafał Grzelczak - will encourage you to check it out. If ‘molecular

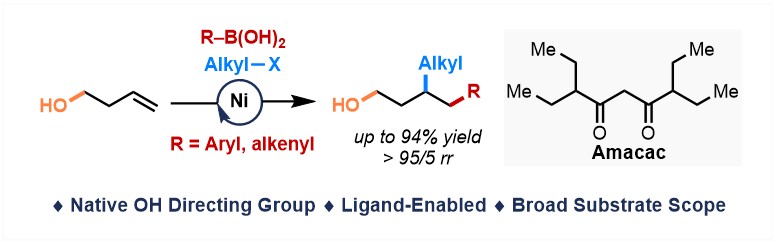
With a bulky acac-type ligand (Amacac), Ni(II)-catalyzed dicarbofunctionalization of alkenyl alcohols has been realized using alcohol as the native DG. The final version of this manuscript out in Chem Catalysis Congrats to Li-Qin. Check out: sciencedirect.com/science/articl…

Check out how we made a minuscule motor rotate 24 times in one direction on its own! Huge thanks to all involved, especially Dave Leigh for making this work possible ACS Publications Huakui Liu Axel Troncossi Stefan Borsley Ben Roberts 🇪🇺 Alex Betts pubs.acs.org/doi/10.1021/ja…

An incredible honor to receive award! Thank you so much to the committee, friends, collaborators and Supramolecular Chemistry for recognizing my work. Thrilled to share this achievement with my friend Qing He. Look forward to meeting you all in ISMSC2025, Kyoto

A Catalysis-Driven Dual Molecular Motor | Journal of the American Chemical Society The University of Manchester pubs.acs.org/doi/10.1021/ja…

PhD positions (SNSF) available at the University of Basel (Switzerland🇨🇭)! Join a new research group working on innovative catalytic pathways to produce endergonic/endothermic molecules🔥. For more details: endergo.net/joinus.html & [email protected]



Just out in Angewandte Chemie. Thanks to the coauthors!

Kinetic Analysis of Redox-Neutral Catalytic Mitsunobu Reaction: Dehydration, Kinetic Barriers, & Hopping between Potential Energy Surfaces | Journal of the American Chemical Society Total Synthesis and Methodology Highlights Keith Andrews Stefan Borsley @RoyalSociety Durham Chemistry pubs.acs.org/doi/10.1021/ja…

Now online & open access: Article by Yuchong Yang, Jonathan R. Nitschke & co-workers Cambridge Chemistry Synthesis of covalently linked knotted cage frameworks nature.com/articles/s4416…

Now online: Article by Qiqiang Xie, Peng Liu, Guangbin Dong & co-workers The Dong Lab Peng Liu Group Enantioconvergent carbenoid insertion into carbon–boron bonds nature.com/articles/s4416… ($)