Jula Inrig
@inrigjula
Nephrologist, researcher, mom.
ID: 1093201063841165312
06-02-2019 17:33:39
93 Tweet
227 Followers
339 Following





Link to the latest podcast from the Rare Kidney Disease Scientific Network. First is Brad Rovin. Second is Jon Barratt. Brad H Rovin Jonathan Barratt medicalaffairs.travere.com/rkd-show/

In our latest GN in Ten episode, Koyal Jain and Kenar Jhaveri chat with Jula Inrig about endothelin, sparsentan, the art & science of clinical trial design, clinical research careers outside academia, and how we got to this "golden era" of neph trials. eu1.hubs.ly/H08RsYt0

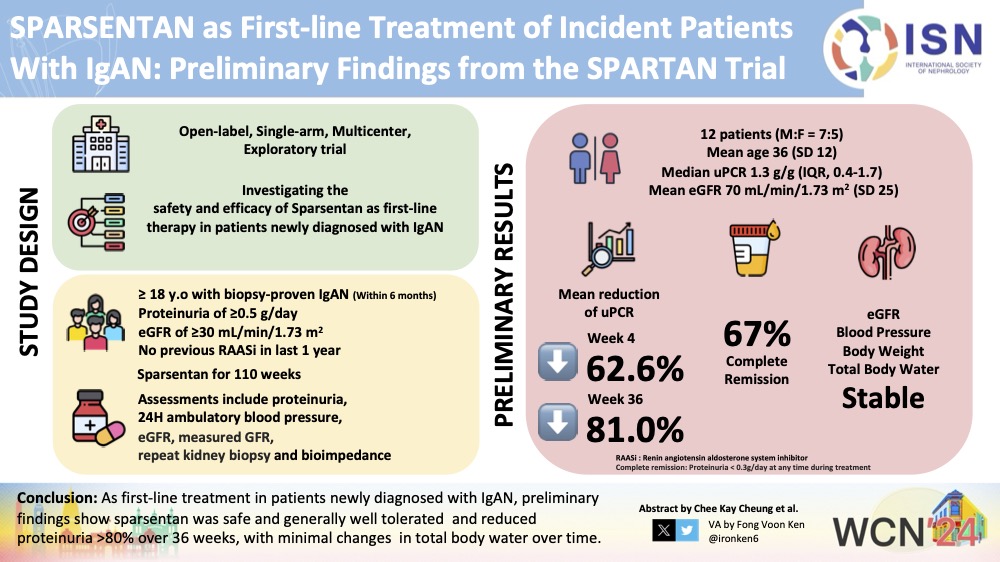
"SPARSENTAN as First-line Treatment of Incident Patients With IgAN: Preliminary Findings from the SPARTAN Trial" by Sian Griffin 🏴 🌻 🇺🇦 🌻 🏳️⚧️, et al, was presented at WCN'24. Their work was transformed into a visual abstract by #ISNWCN Social Media Team member Fong Voon Ken


We are delighted to share abstracts at National Kidney Foundation #SCM24 on our research in #RKD, including analyses comparing the effect of sparsentan as seen in the Phase 3 PROTECT Study in slowing kidney function decline versus RASi in real-world use and in clinical trials in patients with #IgAN.


“There’s always a little love for the glomerulus,” said Jula Inrig, M.D., CMO of Travere Therapeutics during a podcast with the International Society of Glomerular Disease. Jula talks about her career, Kidney Health Initiative, endothelin physiology, and clinical trials of sparsentan in IgAN and FSGS






Today we announced that we’ll present 11 abstracts at the upcoming American Society of Nephrology #ASN24 #KidneyWk in San Diego, CA, later this month on our work in #RareKidneyDisease. #InRareForLife bit.ly/4eUMh7A



We’d like to extend a huge thank you to American Society of Nephrology and to everyone who joined us at #ASN24 #KidneyWk. It was a pleasure coming together and sharing our clinical data and research in #IgAN and #FSGS. Thank you to all those who contributed to another thought-provoking and inspiring