Program On Regulation, Therapeutics, And Law
@portal_research
Division of Pharmacoepidemiology, @BrighamWomens @HarvardMed • Director @akesselheim • Tweets by @bnrome @wbfeldman
ID: 2848609924
http://www.PORTALresearch.org 28-10-2014 18:19:11
4,4K Tweet
2,2K Followers
1,1K Following

NOVEMBER 15: join us for 'Improving Im/migrant Health Care Access: Moving In the Right Direction?' with Program On Regulation, Therapeutics, And Law and Petrie-Flom Center. 🩺 Register here: lp.constantcontactpages.com/ev/reg/ysrb833


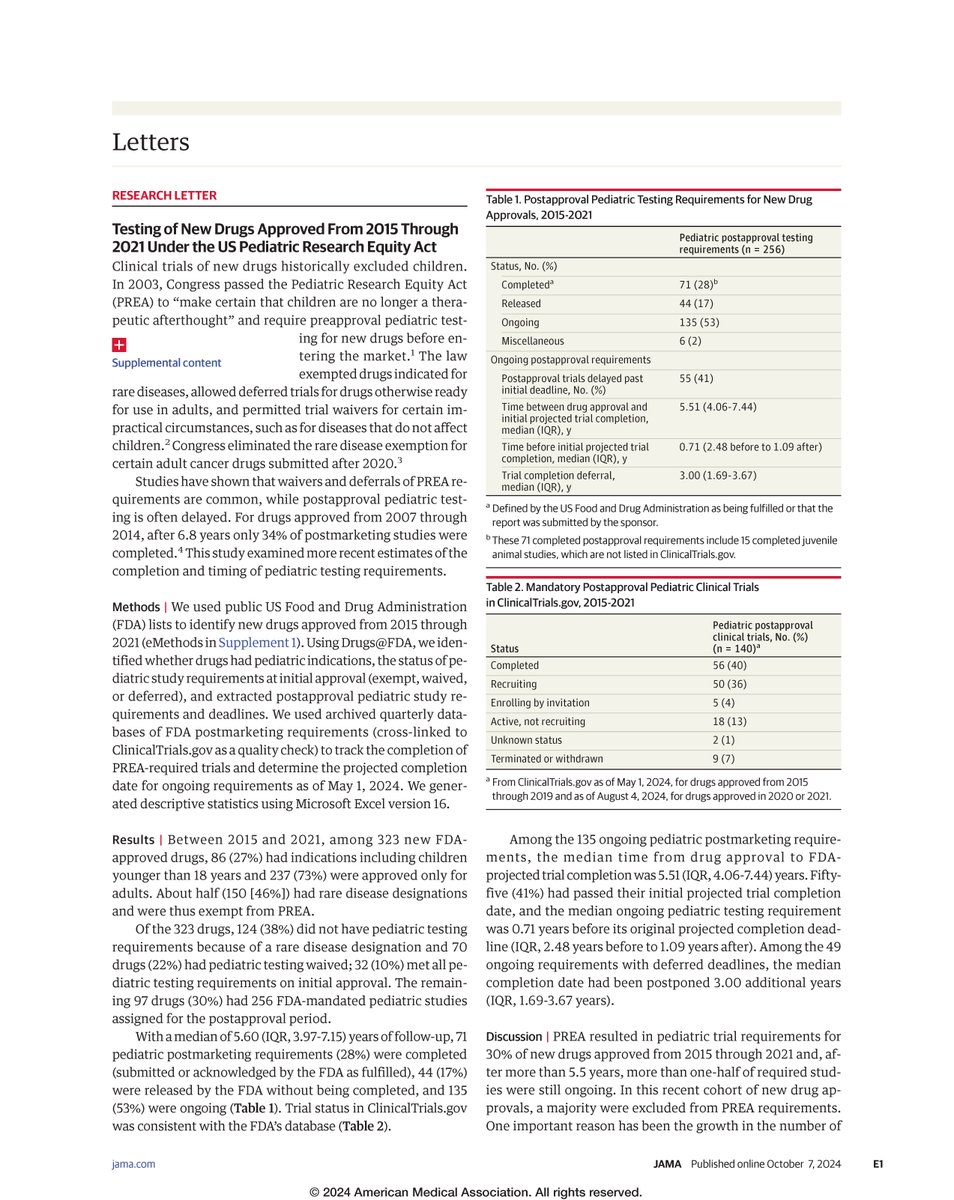
Just out NEJM, Joseph Daval Aaron Kesselheim & I discuss strategies to reduce already-delayed confirmatory trials for accelerated approval drugs. In particular we ask: should the FDA use fines to encourage timely trial completion? nejm.org.acs.hcn.com.au/doi/full/10.10… #medtwitter






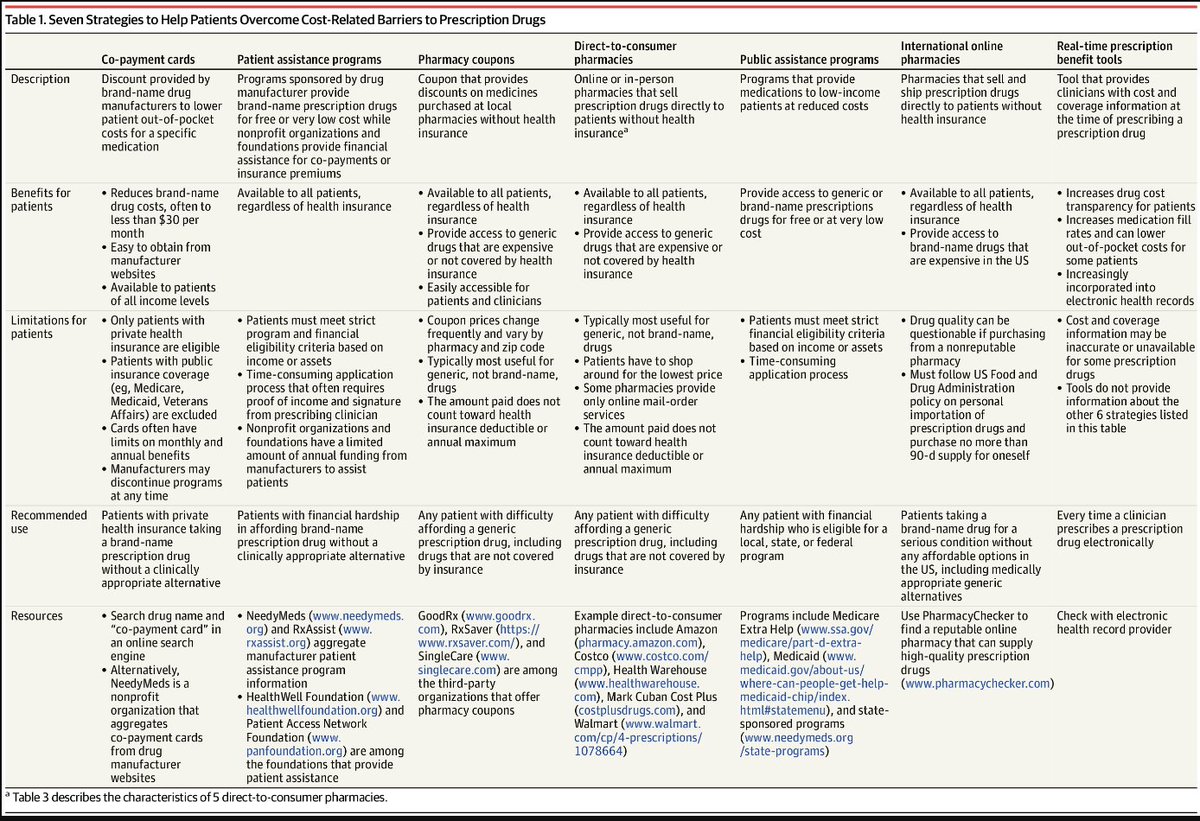
Interested in the strategies and tools clinicians can use to help their patients afford prescription drugs? Check out this new overview in @JAMA by Hussain Lalani, MD, MPH, MSc, Catherine Hwang, Ben Rome, and Aaron Kesselheim highlighting the options available to patients.


Presenting some work on QALY-based cost-effectiveness analyses at #SMDM24 that shows some arguments against the use of QALYs have little empirical justification. Program On Regulation, Therapeutics, And Law


Published today in JAMA Oncology with Aaron Kesselheim and Program On Regulation, Therapeutics, And Law team. We discuss the case of cancer drug Xtandi whose early stage developments were heavily funded by government grants and public funds but now is priced more in the U.S. than in other high-income countries.



Happening now: Ways and Means Committee hearing on lowering costs for patients: The health of the biosimilar market featuring Program On Regulation, Therapeutics, And Law’s Aaron Kesselheim. Watch here: bit.ly/3YqiXzV

Excited to get this brand new book by Jerry Avorn from Program On Regulation, Therapeutics, And Law in the mail today! Can't wait to give it a read! Brigham and Women's Hospital Harvard Medical School