
Sarlah Lab
@sarlahlab
Complex Molecules. Enabling Synthesis.
ID: 1120716191243354112
https://www.sarlahgroup.com 23-04-2019 15:48:56
139 Tweet
5,5K Followers
1,1K Following

Thank you Organic Process Research & Development! We truly appreciate and like your manuscripts, and also use them for the training of the next generation of scientists. sarlahgroup.com/process-of-the…

Check the story behind our Darobactin #synthesis, hosted by Synthesis Workshop. #TotalSynthesis #MerckChemistry

Our last issue of the year contains this great review from David Sarlah Sarlah Lab & co Chemistry at Illinois on dearomative logic in natural product total synthesis Be sure to check out the full review on our website below🔽 pubs.rsc.org/en/content/art…

π-Extended Rubrenes via Dearomative Annulative π-Extension Reaction Nagoya University Itami Lab Sarlah Lab University of Illinois Chemistry at Illinois #PiExtended #Rubrenes #Dearomative #Annulative #PiExtension pubs.acs.org/doi/10.1021/ja…

Featured in our One year of Nature Synthesis collection nature.com/articles/s4416… Article by Sarlah Lab Peng Liu Group Synthesis of (+)-ribostamycin by catalytic, enantioselective hydroamination of benzene

It’s here! Check out episode 4 of this season where we talk #TotalSynthesis of Darobactin A, a project in collaboration with. Sarlah Lab. Check out the show on your favorite podcast platform! Apple: podcasts.apple.com/us/podcast/pha… Spotify: open.spotify.com/episode/5mvFJi…
#TotalSyntheses of Scabrolide A and Yonarolide by Roberto Serrano, Yaroslav D. Boyko Yaroslav Boyko, Lucas W. Hernandez, Aleksandras Lotuzas Aleksandras (Alek) Lotuzas, and David Sarlah Sarlah Lab at Chemistry at Illinois in J. Am. Chem. Soc. pubs.acs.org/doi/10.1021/ja…
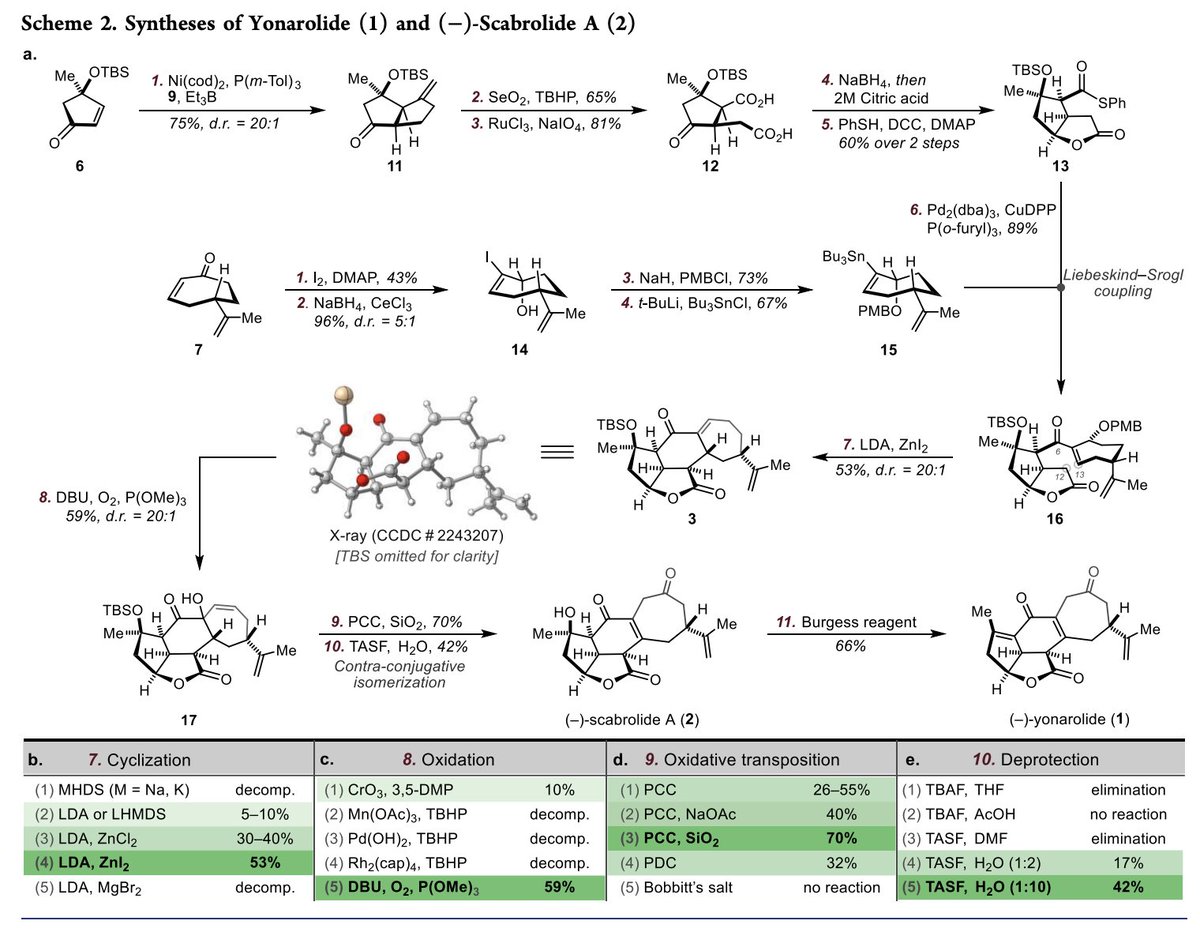

Total Syntheses of Scabrolide A and Yonarolide Yaroslav Boyko Aleksandras (Alek) Lotuzas Sarlah Lab Chemistry at Illinois University of Illinois Total Synthesis and Methodology Highlights #Scabrolide #Yonarolide #Diterpenes #NiPpentannulation #Methylenecyclopropane #Fragmentation #LiebeskindSroglCoupling pubs.acs.org/doi/10.1021/ja…

It will fit but won't flip! Check out our preliminary collaborative study with SpiroChem AG and #MerckChemistry, evaluating (very!) rigid Dewar pyridine derivatives as programmable piperidine isosteres. ift.tt/Bn4Kape


Day 3 ACS Organic Division #GRS2023 Amazing student presentations: Alexander Oanta (Northwestern Univ), David Ryffel (Univ of Illinois), Jenna Humke (Univ of Minnesota), Matthew McVeigh (UCLA) Sarlah Lab Garg Lab UCLA


David Sarlah (Sarlah Lab) closing the day at #EFMCASMC23 (with homefield advantage in #Croatia): Larock cyclization strategy based atropselective synthesis of darobactin A & even more fun with unhealthy looking twisted tricyclic terpenes. Next stop: speaker‘s dinner!


Congratulations to our former member Conghui Tang for an awesome paper!


Oxidative Dearomatization of Pyridines | Journal of the American Chemical Society Genomic Biology Sarlah Lab University of Illinois Total Synthesis and Methodology Highlights #Dearomatization #Pyridines pubs.acs.org/doi/10.1021/ja…

Now online: News & Views on the Article by Wen-Xiu Xu, Zhuo Peng, Qing-Xiu Gu, Yao Zhu, Li-Han Zhao, Fucheng Leng & Hai-Hua Lu nature.com/articles/s4416… ($) Hydrogenation streamlines cyclolignan synthesis by David Ryffel & David Sarlah Sarlah Lab nature.com/articles/s4416… ($)


Total Syntheses of Scabrolide B, Ineleganolide, and Related Norcembranoids | Journal of the American Chemical Society Sarlah Lab University of Illinois Chemistry at Illinois Rice University Total Synthesis Total Synthesis and Methodology Highlights #Scabrolide #Norcembranoids #Mukaiyama #Michael pubs.acs.org/doi/10.1021/ja…



Stereodivergent #Synthesis of Perhydrobenz[e]indene Terpenoids by Cheng Yang, Christopher J. Huck, Yaroslav Boyko, Shang Ning, Uroš Vezonik, Alexander S. Shved, Scott E. Denmark, Binh Khanh Mai, David Sarlah Sarlah Lab at Rice University Chemistry Department in J. Am. Chem. Soc. pubs.acs.org/doi/10.1021/ja…
![Total Synthesis (@totalsynthesis) on Twitter photo Stereodivergent #Synthesis of Perhydrobenz[e]indene Terpenoids by Cheng Yang, Christopher J. Huck, <a href="/YaroslavBoyko4/">Yaroslav Boyko</a>, Shang Ning, Uroš Vezonik, Alexander S. Shved, Scott E. Denmark, Binh Khanh Mai, David Sarlah <a href="/SarlahLab/">Sarlah Lab</a> at <a href="/RiceChemistry/">Rice University Chemistry Department</a> in <a href="/J_A_C_S/">J. Am. Chem. Soc.</a> pubs.acs.org/doi/10.1021/ja… Stereodivergent #Synthesis of Perhydrobenz[e]indene Terpenoids by Cheng Yang, Christopher J. Huck, <a href="/YaroslavBoyko4/">Yaroslav Boyko</a>, Shang Ning, Uroš Vezonik, Alexander S. Shved, Scott E. Denmark, Binh Khanh Mai, David Sarlah <a href="/SarlahLab/">Sarlah Lab</a> at <a href="/RiceChemistry/">Rice University Chemistry Department</a> in <a href="/J_A_C_S/">J. Am. Chem. Soc.</a> pubs.acs.org/doi/10.1021/ja…](https://pbs.twimg.com/media/GvQzlnvWwAAexh0.jpg)
