
The Colonna Lab
@thecolonnalab
We study innate immunity with a focus on ILCs, macrophages, and DCs. The account is led by trainees. @wusm_pathology @WUSTLmed
ID: 1387204703969325056
http://sites.wustl.edu/colonnalab 28-04-2021 00:40:55
82 Tweet
3,3K Followers
110 Following

Thrilled to share our work has been highlighted by Dr Monica M. Bertagolli @NIHDirector The Colonna Lab

Excited to share our latest paper: combining anti–PD1 treatment with TREM2 deficiency in mice triggers a proinflammatory response in the gut, promoting the expansion of Ruminococcus gnavus. Congrats to first authors Blanda Di Luccia Martina Molgora Darya Khantakova doi.org/10.1126/sciimm…

Happy to share our new study showing IL-22BP deficiency enhances gut microbiota's defense against infections. This boosts acetate production by Il22ra2–/–associated microbiota, suggesting IL-22BP as a therapeutic target. Led by José Fachi, PhD and Blanda Di Luccia pnas.org/doi/10.1073/pn…

Congrats Patrick Rodrigues! Distinct lineages contribute to cDC2 diversity: lymphoid-derived pDC-like and myeloid-derived pre-cDC2 both develop into transcriptionally similar cDC2, crucial for humoral immune responses, and are distinct from pro-DC3 derived DC3. twtr.to/Tr5-h
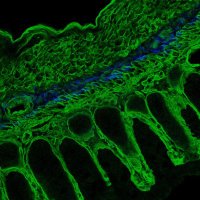
Collaboration with The Colonna Lab & others, in press at Developmental Cell: The FGFR1OP gene, implicated in #Crohns, contributes to intestinal barrier function by modulating the actin cytoskeleton in crypt cells. (1/2) cell.com/developmental-…

Our new paper led by Tihana Trsan, collaboration with Xavier Lab, investigating the link between FGFR1OP and Crohn’s disease. Deletion of FGFR1OP, a centrosomal protein, in mouse gut cells disrupts crypt architecture, causing inflammation and fatality cell.com/developmental-…

Check out our newest resource led by Natalia Jaeger and Alina Ulezko Antonova describing the NK-ILC1 gradient in human tissues: rdcu.be/dM64F. The IEL contains true mature (PRDM1+) and immature (PRDM1-) EOMES-ILC1, while other tissues harbor ILC1-like NKs expressing ZNF683.

🧵 Excited to share my first preprint from my PhD Kipnis Lab and The Colonna Lab: "Brain-Engrafted Monocyte-derived Macrophages from Blood and Skull-Bone Marrow Exhibit Distinct Identities from Microglia." 🧠🦴 biorxiv.org/content/10.110…


Our work by Fachi et al. José Fachi, PhD is out in PNAS! We show how NKp46+ ILC3s boost early gut defense against C. difficile infection by producing GM-CSF to support neutrophils. A potential path to new CDI treatments! pnas.org/doi/10.1073/pn…


Excited to share that our work is featured on the cover of the latest issue of Cell Host & Microbe! Check it out! The Colonna Lab Marco Vinolo The Pew Trusts #Microbiology #Immunology #CellHostMicrobe

Loss of ATG7 in microglia impairs UPR, triggers ferroptosis, and weakens amyloid pathology control rupress.org/jem/article-ab…, in this work we linked autophagy with UPR and ferroptosis in the microglial response to AD Zhangying Cai The Colonna Lab 🥳🍻🆗

Cai et al. The Colonna Lab WashU Medicine show that, in an #Alzheimers mouse model, microglial Atg7 deletion impairs plaque coverage, increasing Aβ diffusion & neurotoxicity, linked to reduced UPR, increased oxidative stress, & #ferroptosis of #microglia hubs.la/Q036BHM10



Congratulations to physicist Carl Bender of WashU Arts & Sciences and immunologist Marco Colonna WashU Medicine, who have been elected to the American Academy of Arts & Sciences for their pioneering work in quantum theory and immune pathways in Alzheimer’s. bit.ly/42SK87N

Congrats to Ranit Kedmi’s group! RORγt⁺ APCs prime food-specific pTregs and induce oral tolerance. Infection or food poisoning temporarily bypasses this, allowing CD8αβ T cell responses to mimicked food antigens without breaking long-term oral tolerance. nature.com/articles/s4158…
