
Charlene Liao
@charlene_liao
Founder, President and CEO
Immune-Onc Therapeutics, Inc.
ID: 919596436508905472
http://www.Immune-Onc.com 15-10-2017 16:10:50
262 Tweet
159 Followers
69 Following

Another great recognition for Immune-Onc Therapeutics ! I am immensely proud of our team for their commitment to making a difference in the lives of patients and I am grateful to the S.F. Business Times Multimedia for shining a spotlight on the vital role of women leaders in the life sciences industry.


I’m excited to announce that Immune-Onc Therapeutics will present new data on IO-202 in #CMML at the #EHA2024 conference in Spain next month. If you plan to attend #EHA2024, please get in touch to say “Hello” and learn more about our work in blood cancer. I look forward to seeing you there!


Thank you John Carroll for writing about your journey as a cancer patient and for shedding light on Merkel cell carcinoma (MCC) and the perseverance of drug developers to get 3 new medicines approved that mobilize our immune system to fight this rare cancer. We have more to come!



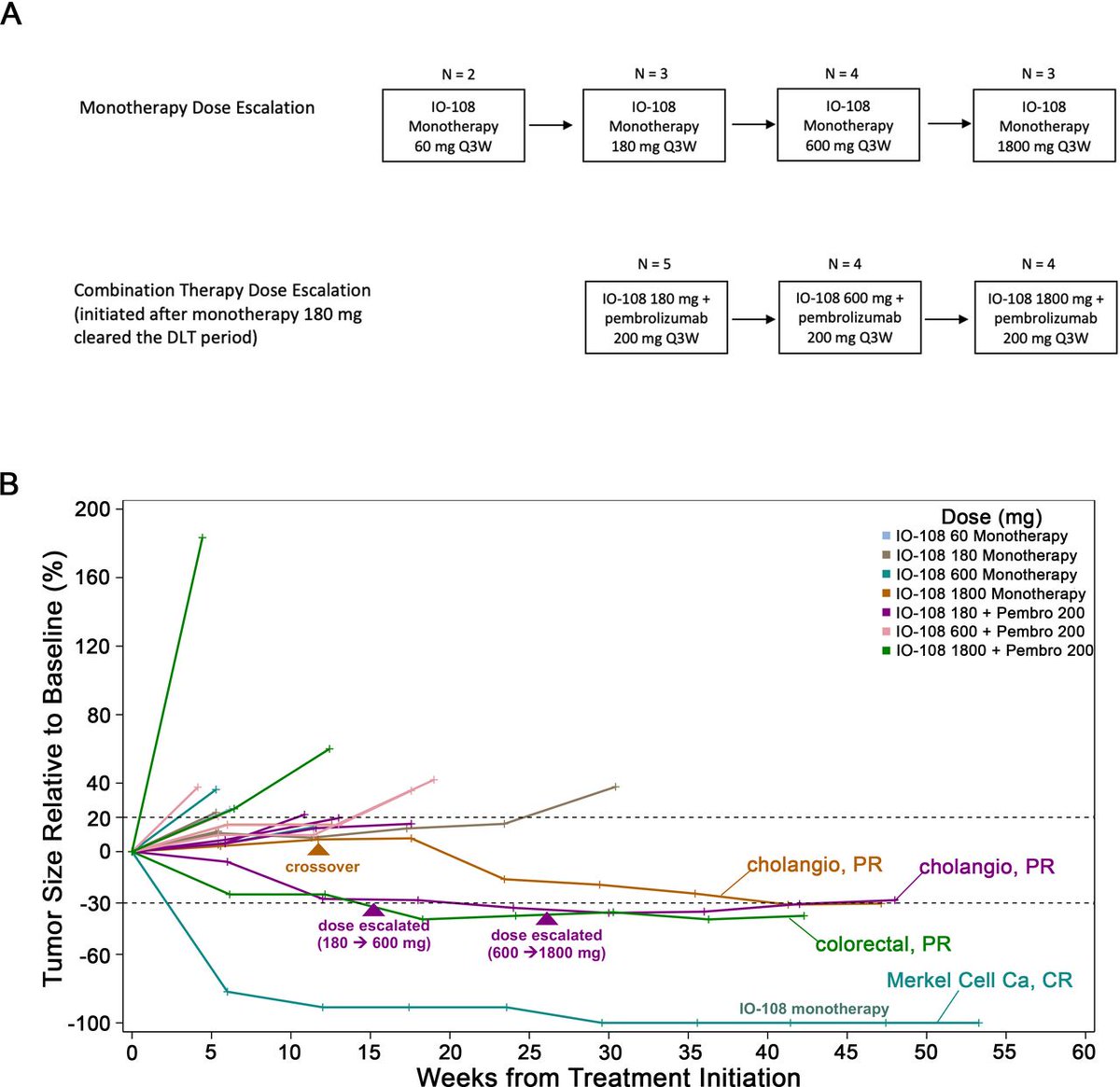
New #JITC article: Phase I dose escalation study of IO-108, an anti-LILRB2 antibody, in patients with advanced solid tumors bit.ly/3ZgH0SF Aung Naing


Our Phase 1 data on IO-108 was published in Journal for ImmunoTherapy of Cancer · Durable responses in advanced solid tumors · Promising monotherapy & combo results with pembrolizumab · Potential to overcome resistance to checkpoint inhibitors bit.ly/3ZgH0SF

Immune-Onc Therapeutics will present important updates on its Phase 1b data on IO-202 for CMML. Don’t miss the oral presentation at #ASH2024 on Monday!






This is an important step for Immune-Onc Therapeutics and for patients with #HCC. We look forward to learning how IO-108 may add to existing therapies and provide more choices for #patients. I’m very grateful to our team, our partners at Roche, and clinical investigators leading this effort.

The publication of our IO-202 Phase 1 data in Blood Neoplasia marks an important moment for Immune-Onc Therapeutics and for patients with HMA-naïve #CMML and monocytic #AML, who urgently need better options. We are grateful to the patients, investigators, care teams and staff for this work.
