Hari B. Keshava
@hari_keshava
Thoracic Surgeon @ucirvinesurgery @valongbeach | Marathon Runner #runningsuture | Engineer to MD | Surgeon and Patient Advocate
ID: 2909651926
http://www.ucihealth.org/find-a-doctor/k/hari-b-keshava 07-12-2014 18:12:42
5,5K Tweet
2,2K Followers
1,1K Following


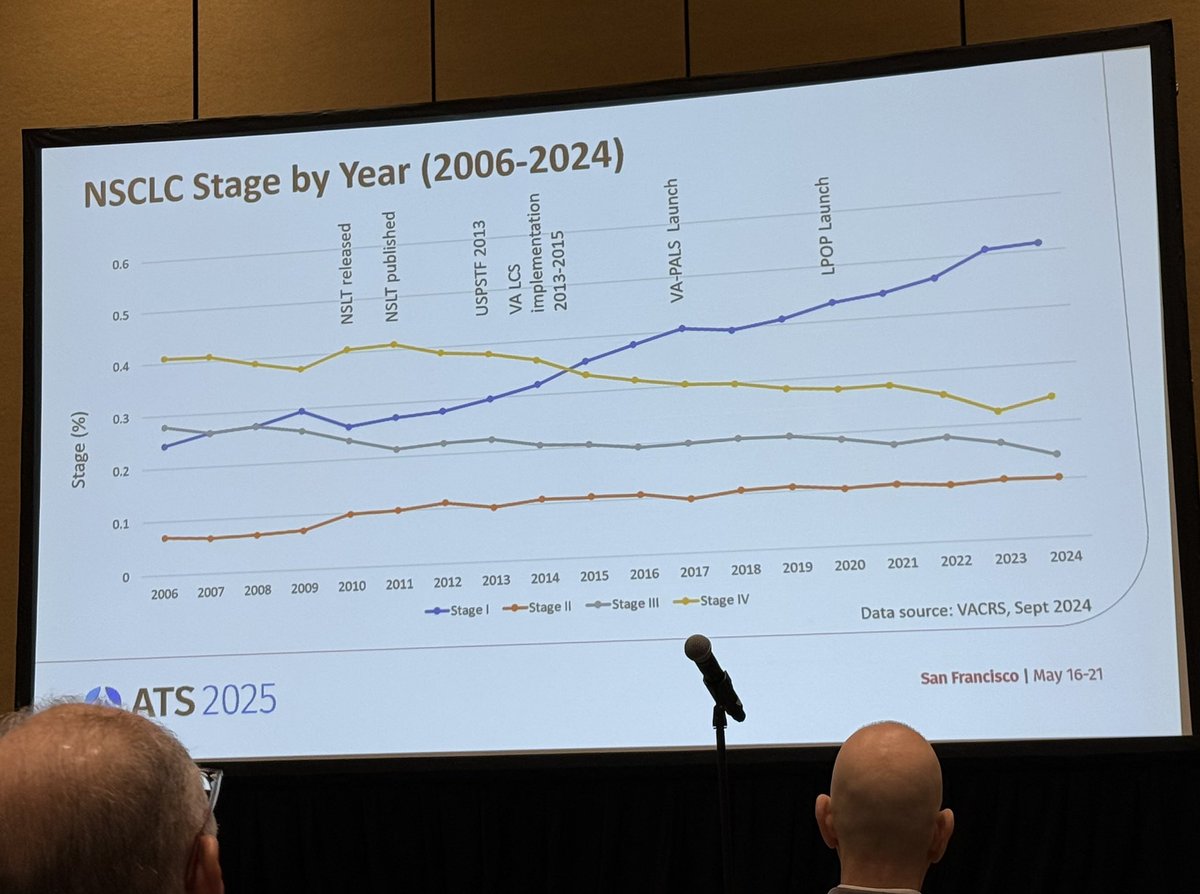
🫁 The VA continues to lead the most successful lung screening program ever implemented in the US. These data confirm it’s saving lives as demonstrated in the yellow line representing unprecedented declines in stage IV cases. #ATS2025 VA Research House Committee on Veterans' Affairs Veterans Affairs

The 'Omega' valve and a new thought process in the management of reflux. Amazing to hear from our chairman and thought leader about Reflux! ninh nguyen The American Foregut Society UC Irvine Surgery #reflux #foregut #gerd





Won't be at ASCO this year, but I'm excited to see/hear about the studies and read the Twitter debates!!! Sandip Patel MD FASCO Narjust Florez, MD, FASCO Misako Nagasaka Drew Moghanaki Brendon Stiles Stephen V Liu, MD Linda Martin Patrick Forde Jonathan Spicer MD PhD #asco2025

Hari B. Keshava Sandip Patel MD FASCO Narjust Florez, MD, FASCO Misako Nagasaka Drew Moghanaki Brendon Stiles Stephen V Liu, MD Linda Martin Patrick Forde I recommend checking out the CM816 OS, the outcomes by molecular profile from 77T, the first report of NeoADAURA findings and the complete data on arms 1, 2 and 4 of NeoCOAST2! Lots of great data that will inform practice and the next generation of phase 3 trials!






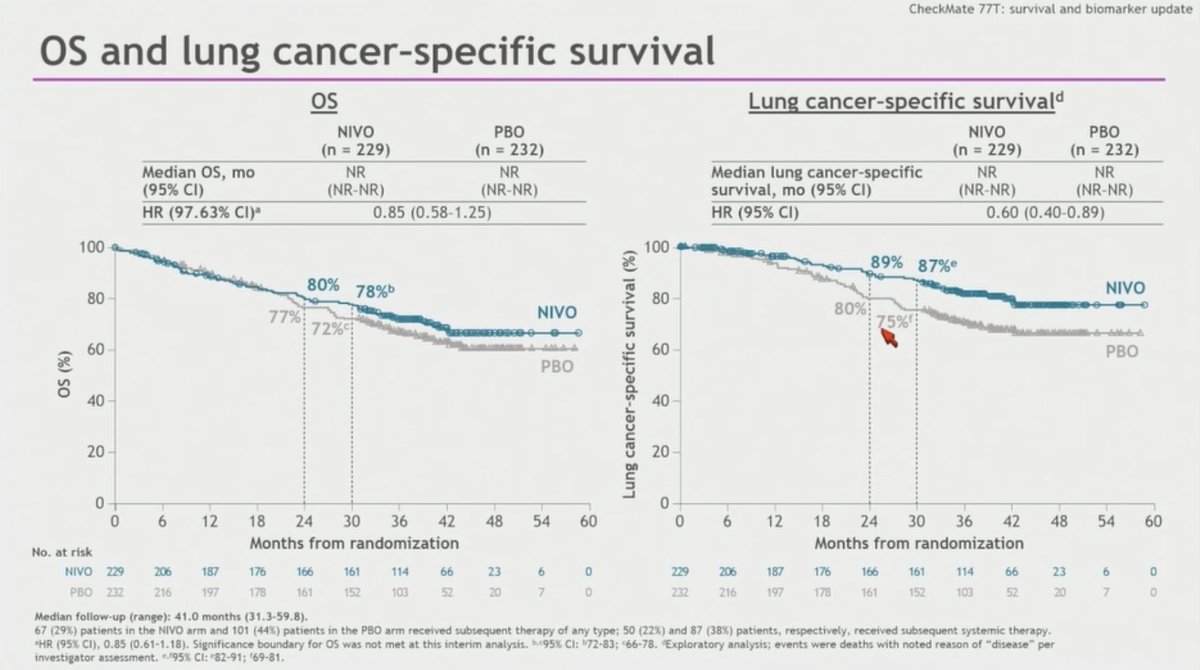
#ASCO25 update on CheckMate 77T: Periop nivo vs plb in resectable NSCLC (mFUP 41 mo) M.Provencio 🔹 EFS: 46.6 vs 16.9 mo (HR 0.61), 30-mo 61% vs 43% 🔹 Benefit regardless of KRAS, STK11, KEAP1, PD-L1 status 🔹 ctDNA/pCR linked to EFS 🔹 OS NR trend HR 0.85 (30mo 78% vs 72%)





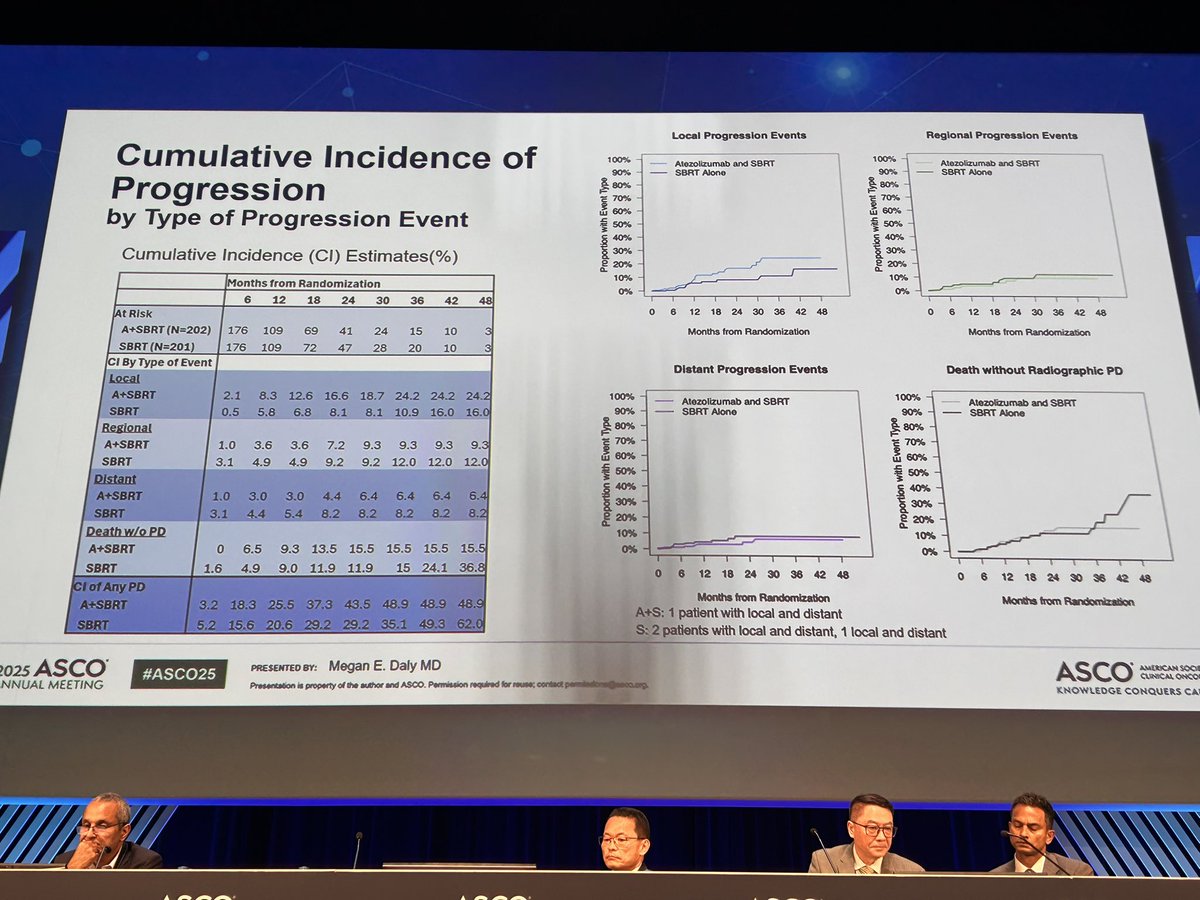
Dr. Megan Daly presents at #ASCO25 the SWOG Cancer Research Network S1914 Phase 3 study. Unfortunately, SBRT + atezolizumab failed to improve PFS or OS over SBRT in early stage unresectable/inoperable high-risk NSCLC. ⭐️Stopped early for futility ⭐️PFS 1.85, OS 1.76 for SBRT+atezo ⭐️⬆️local POD