Binh Khanh Mai
@khanhbinh183
Computational chemist
ID: 321424716
https://sites.google.com/site/khanhbinh183 21-06-2011 15:12:53
114 Tweet
326 Followers
647 Following
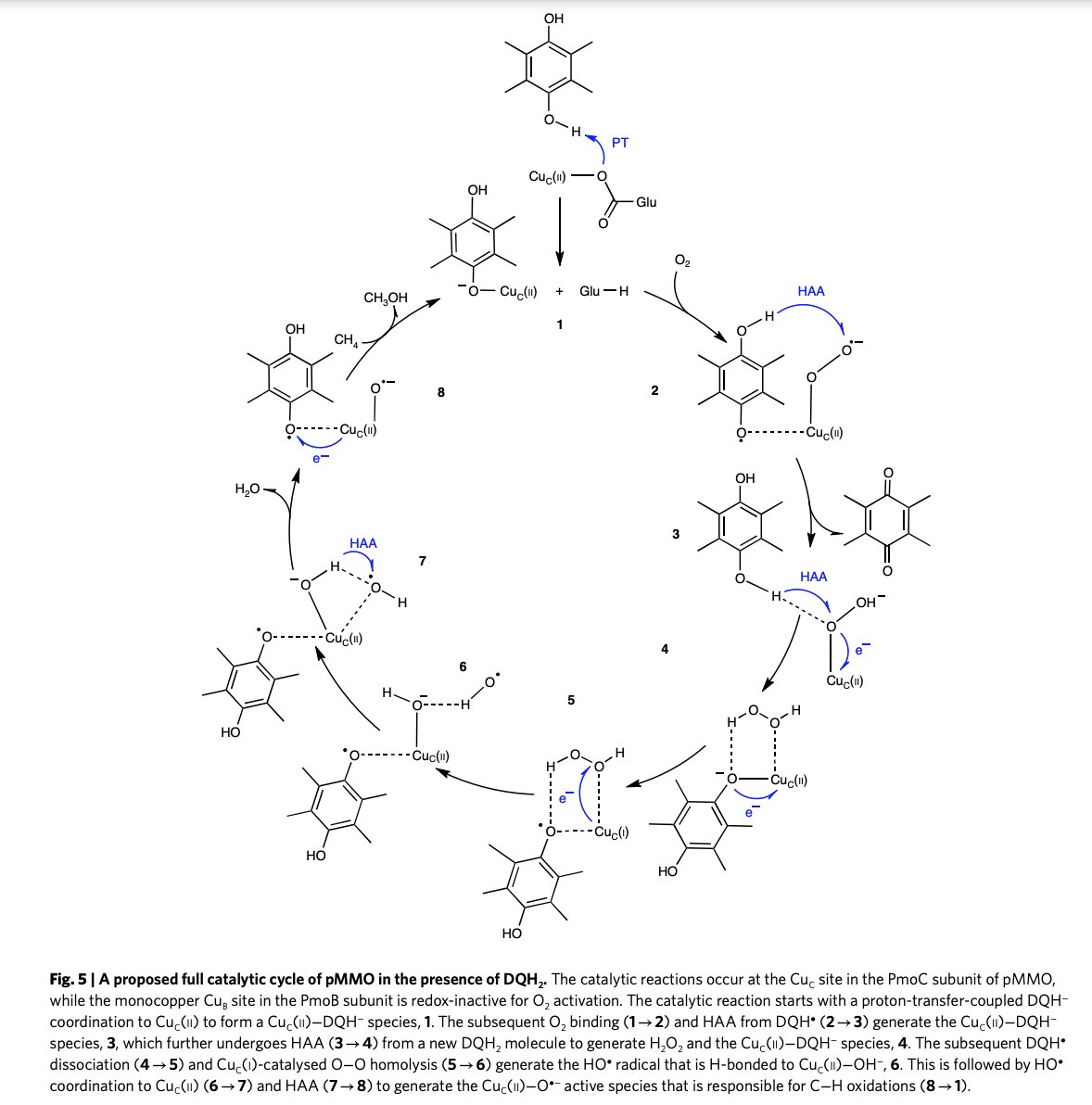
Deciphering the oxygen activation mechanism at the CuC site of particulate methane monooxygenase by Wei Peng, Xiaoyang Qu, Sason Shaik , and Binju Wang in Nature Catalysis nature.com/articles/s4192…

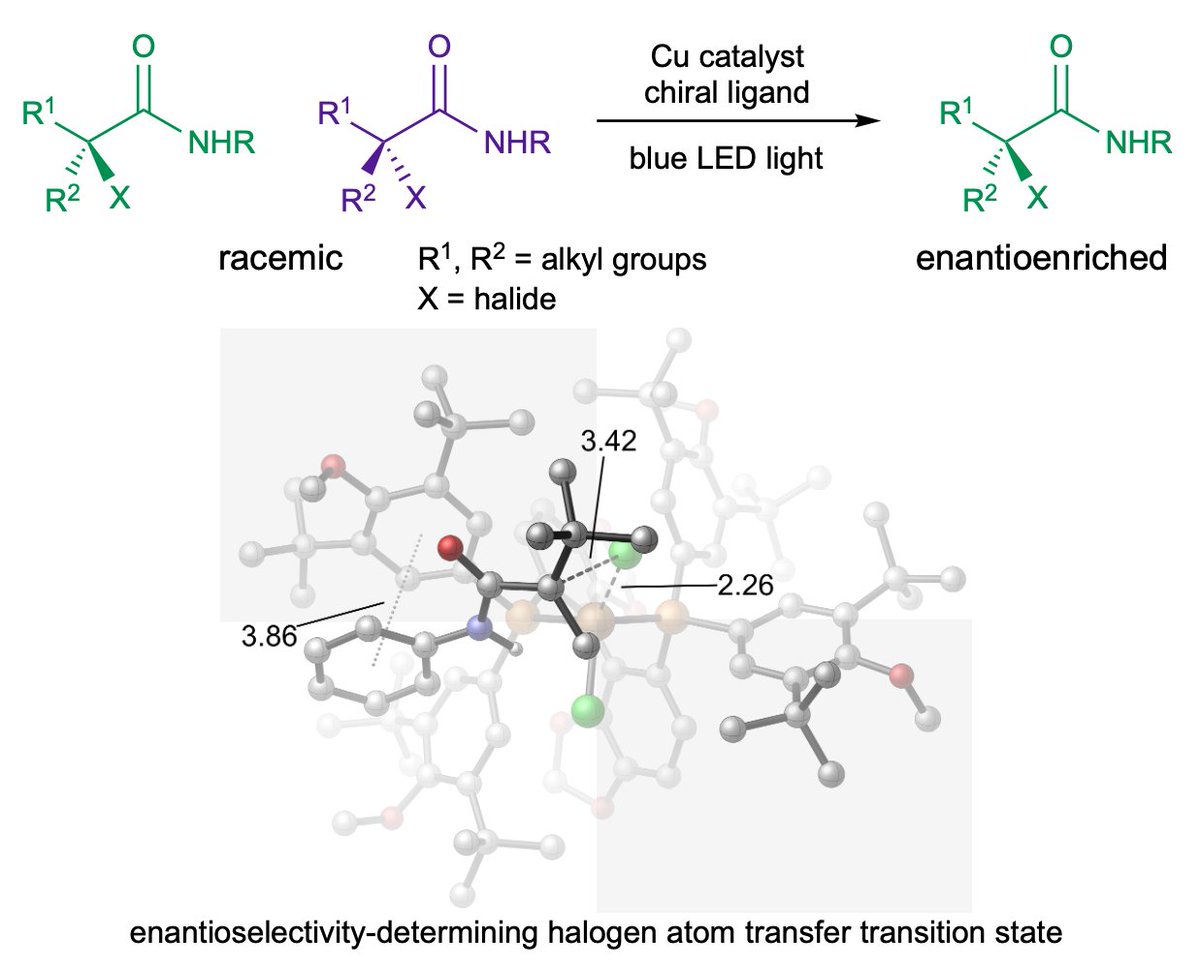
Congrats Shaozhen Nie, Alex, and Erin Kuker--so proud of your efforts! Check out our latest idea for making enantiopure sulfides J. Am. Chem. Soc. UCI Chemistry: pubs.acs.org/doi/10.1021/ja…


Playlists for introductory online lectures by 🚀 Anders Christensen and Guido von Rudorff given last spring online University of Basel: Python youtube.com/playlist?list=… Numerics: youtube.com/playlist?list=… Machine learning: youtube.com/playlist?list=… via YouTube #compchem

Hey chemtweeps, please help to spread the word. We have a postdoc position opening in my lab CMU Chemistry. Focus on dev ML and QM algorithms for #compchem & chemical reactivity. Chemjobber #CompChemJobs #ChemJobs ChemPostdocBot #chempostdoc apply.interfolio.com/89604
We are excited to share our latest work with Drs. Craig Stivala & Jason Zbieg of Genentech and Dr. Binh Mai of Peng Liu Group on the asymmetric allylation of nitroalkanes. Congrats to Woo Ok, Brian, Zach, and Seung Wook! J. Am. Chem. Soc. pubs.acs.org/doi/10.1021/ja… (1/2)




Congratulations to TJ and our collaborators in Peng Liu Group on the recent paper in J. Am. Chem. Soc. describing an enantioselective beta-fluoride elimination. pubs.acs.org/doi/full/10.10…


Challenging Asymmetric C=O 1,2-Addn Using Vinyl Heteroarene Pronucleophiles: Regiodivergent Processes through a Dearomatized Allyl–Cu Species MIT Chemistry Massachusetts Institute of Technology (MIT) Buchwald Group Pitt Chemistry #Catalysis University of Pittsburgh Total Synthesis and Methodology Highlights #Enantioselective pubs.acs.org/doi/10.1021/ja…

Check out our latest publication in J. Am. Chem. Soc. w/ Yang Yang @UCSB_chemistry Our study suggests radical rebound, rather than C-H abstraction, is enantioselectivity-determining in a P411-catalyzed C-H amination developed by the Frances Arnold group. pubs.acs.org/doi/10.1021/ja…



Check out postdoc Dong Research Group's new report on the enantioselective hydromethylation of olefins! The collaboration with Peng Liu Group is now out in John Callahan pubs.acs.org/doi/10.1021/ja…

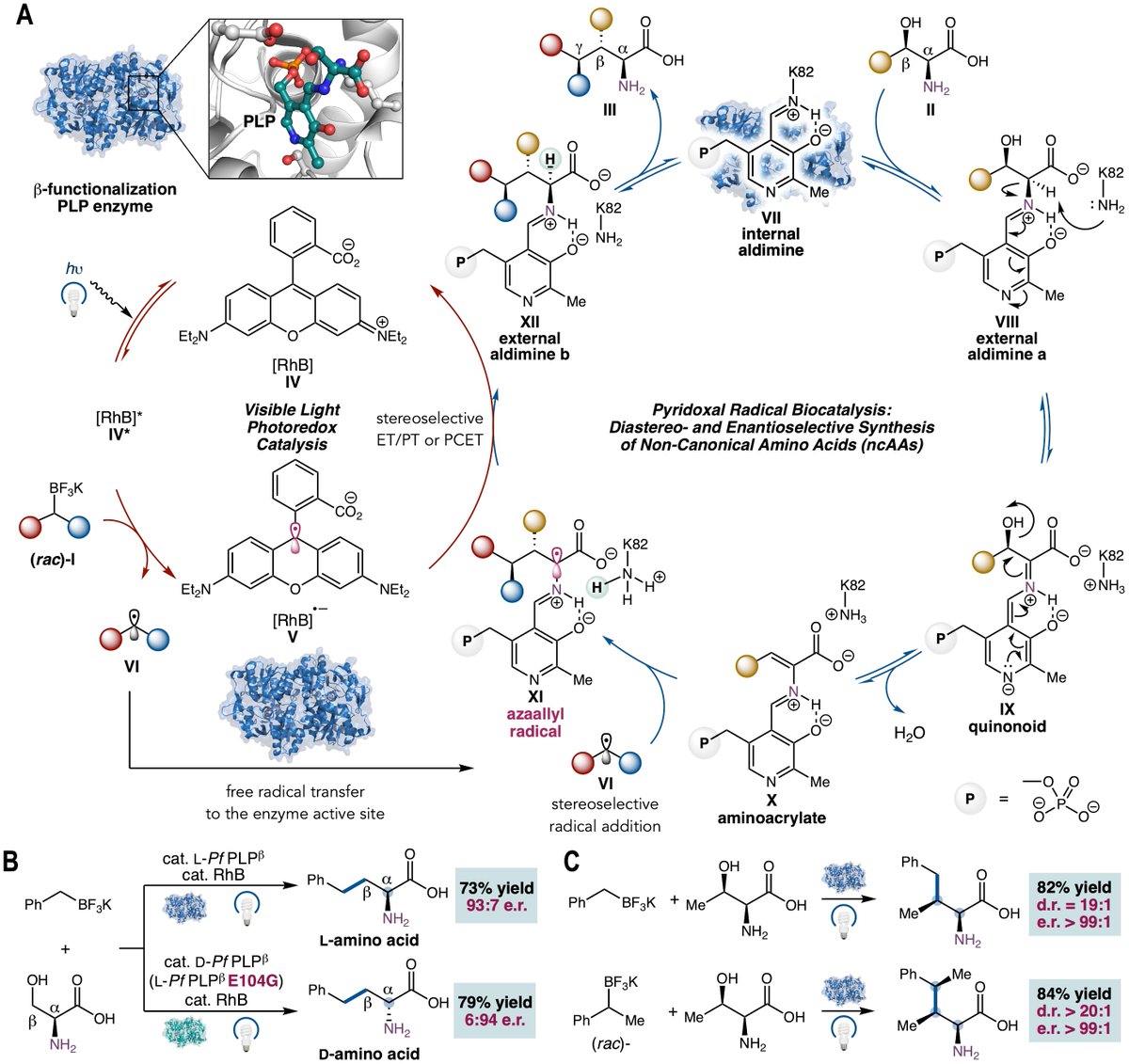
We published our first paper on pyridoxal radical biocatalysis in Science Magazine : lnkd.in/gkB5dpKM. What we are most excited about is the ability to design general biocatalytic activation modes which are both new-to-biology and new-to-chemistry:

Our paper regarding a catalytic process designed for a programmable strategy for synthesis of a scarce indole alkaloid natural product and related precisely altered frameworks is out in Nature Chemistry! - nature.com/articles/s4155…