Kwangmin Shin
@kwangminshin
Organic chemist
Associate professor of chemistry @SKKU, Korea
Former postdoc @BuchwildGroup
Ph.D. @kaistpr (S. Chang Group)
ID: 1549275794190761984
https://www.kmshinlab.com/ 19-07-2022 06:12:00
184 Tweet
282 Followers
333 Following




Excited to share our work (Eunsung Lee) in collaboration with Prof. Tae-Lim Choi's group! Air-stable ruthenium catalysts with tethered CAAC ligands for REMP. A heartfelt thank you to the reviewers and editor for their invaluable advice! doi.org/10.1021/jacs.4…

Enantioselective Synthesis of α-Aryl Ketones by a Cobalt-Catalyzed Semipinacol Rearrangement (Craig P. Johnston and co-workers) @TheJohnstonLab St Andrews School of Chemistry #openaccess 🔓 onlinelibrary.wiley.com/doi/10.1002/an…

Delighted to contribute some thoughts to this wonderful article in Chemistry World on putting fluorine in drug molecules along with Timothy Noël, Gouverneur Group, David O'Hagan, and Rob Young! Check it out! chemistryworld.com/features/putti…

Our synthesis of the bisnortriterpenoid rubriflordilactone B, which features a [2,3]-Wittig−Still rearrangement, a Friedel−Crafts cyclization and an E1cB reaction/transesterification/oxa-Michael addition cascade, is now out in J. Am. Chem. Soc. : bit.ly/430IyCx

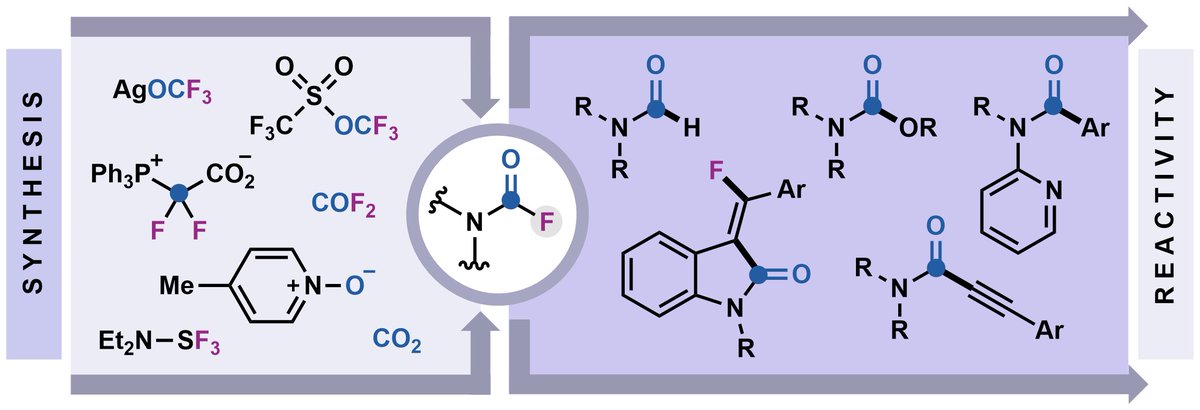
Hot off the press! 🔥We review the use of carbamoyl fluorides as a platform to explore fluoride-enabled reactivity. Just accepted in SYNTHESIS Journal Chemistry at Thieme. Congrats, Maryam! The Le Group thieme-connect.com/products/ejour…





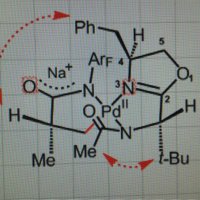
I've been experimenting with the OMol25 models from FAIR Chemistry, and I'm quite happy so far. Here's a challenging system that I worked on with Buchwald Group: Pd-catalyzed C–F reductive elimination. The model gives ∆E‡ ~= 23.2 kcal/mol, vs. a reported ∆G‡ of 20.6!





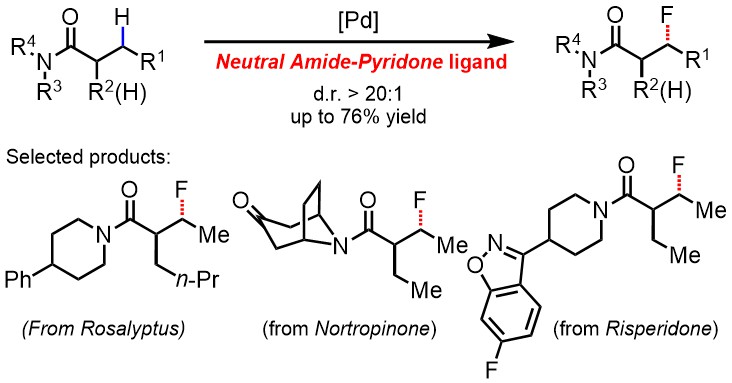
Our third project on alkene hydrofluorination is now published in JOC (J Org Chem/Org Lett )! We showcase the use of diverse nucleophilic fluorine sources for the synthesis of β-fluorinated amides. Congrats to Baeho and Dohyun! pubs.acs.org/doi/10.1021/ac…


Congrats to Yoojin and Taewan for the publication of Total Synthesis of (+)-Herpotrichones A–C | Journal of the American Chemical Society J. Am. Chem. Soc. KAIST Chemistry pubs.acs.org/doi/10.1021/ja…

Kim's research group at JBNU (sites.google.com/site/jbnusynth…) has one postdoctoral position available immediately. Primary hire will be on polymer synthesis and mechanochemistry. Strong candidates in organic synthesis are also considered. Please send CV to [email protected]