Nicole Casasanta
@ncasasanta
Heme/Onc Fellow @YaleHemOnc aspiring breast oncologist | @MSH_MedChiefs @DOMSinaiNYC | alum @GWSMHS @GWtweets
ID: 572072752
05-05-2012 21:06:04
373 Tweet
187 Followers
487 Following


In metastatic #breastcancer, sacituzumab govitecan given after trastuzumab deruxtecan was linked to ⬇️ PFS across all subtypes. Real-world data published in JNCI show differences in outcomes by type. academic.oup.com/jnci/advance-a… Smilow Cancer Hospital YaleBreastCancer Maryam Lustberg MD, MPH, FASCO

Excited to have joined Eric Winer, MD and Etienne for this discussion on medical oncology training, the future of oncology, and more! 🎙️🩺 Yale Hematology Oncology Fellows Yale Cancer Center YaleBreastCancer

Future of Cancer Care and the Next Generation of Oncologists – Yale Cancer Center Yale Cancer Center Yale Hematology Oncology Fellows Müschen Lab Nicole Casasanta, MD Eric Winer, MD oncodaily.com/voices/future-… #OncoDaily #Oncology #Cancer #Health #Medicine #MedX #MedEd #MedNews CancerWorld




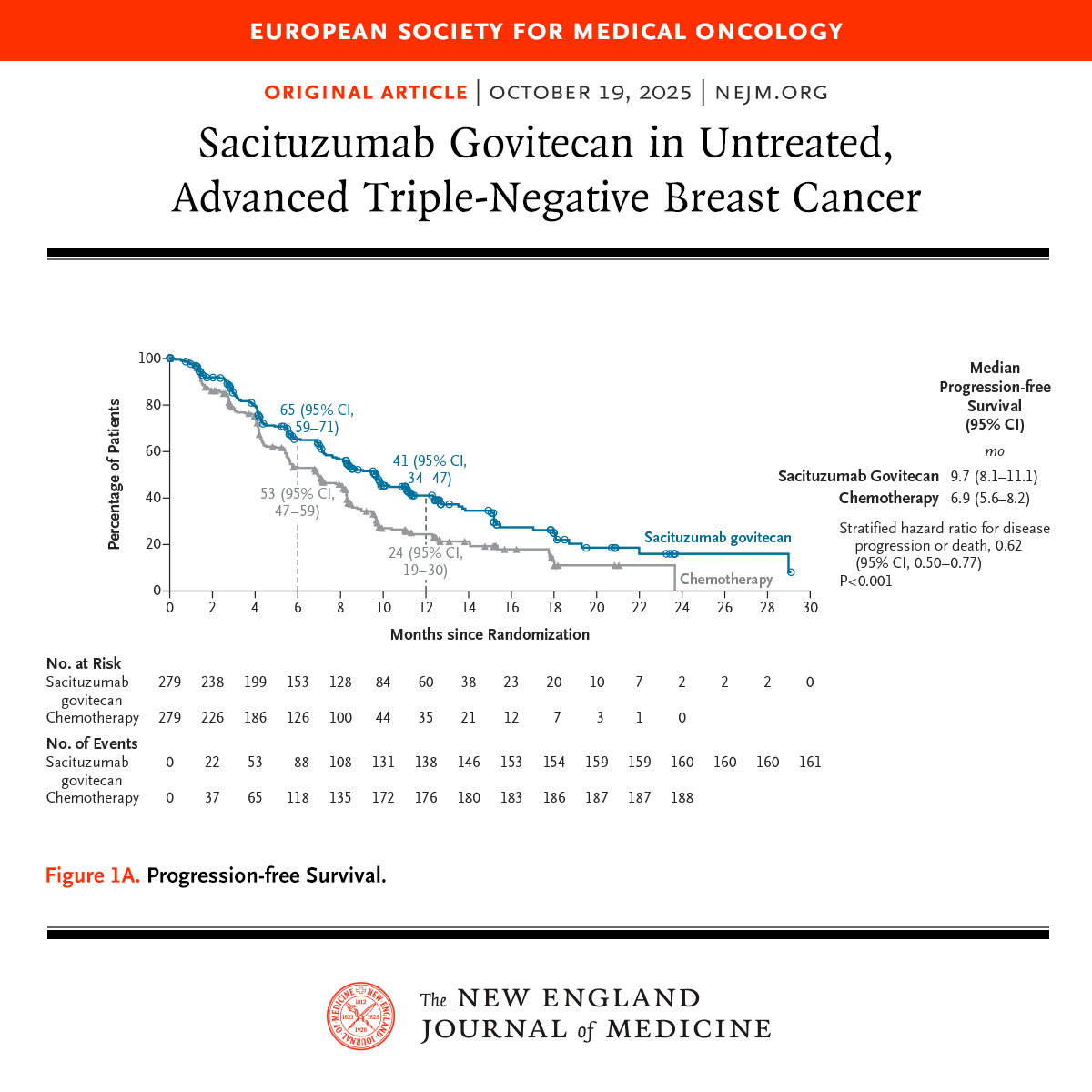
Presented at #ESMO25: In untreated, advanced triple-negative breast cancer, sacituzumab govitecan led to significantly longer progression-free survival than chemotherapy (9.7 months vs. 6.9 months). Full ASCENT-03 phase 3 trial results: nej.md/4ncuUCV ESMO - Eur. Oncology



💡Fabulous first day of the Lynn Sage Breast Cancer Symposium discussing obesity, sustainability, breast reconstruction, pathology, AI in oncology, approaches to management & so much more ✨Thank you Lynn Sage Breast Cancer Foundation for the travel award! 💐#LSBC25 Lurie Cancer Center Stephanie A. Haddad, DO Yale Hematology Oncology Fellows


📍Thought provoking end to the Lynn Sage Breast Cancer Symposium discussing goals of de-escalation in low risk, escalation in high risk, & individualized treatment in intermediate risk. Thankful for the opportunity to attend! ✨#LSBCS25 Lynn Sage Breast Cancer Foundation Lurie Cancer Center Harold J. Burstein, MD, PhD, FASCO