
Ritter Lab
@ritter_lab
Research in the Ritter group focuses on the development of novel reaction chemistry.
ID: 899589682115301376
21-08-2017 11:11:08
364 Tweet
10,10K Followers
136 Following

Late-Stage Diazoester Installation via Arylthianthrenium Salts (Tobias Ritter and co-workers) Ritter Lab #openaccess 🔓 onlinelibrary.wiley.com/doi/10.1002/an…

'How do I thianthrenate X?' – In our new article in J. Am. Chem. Soc., Dilgam Ahmadli presents simple guidelines for selecting reaction conditions and with our collaborators from @syngenta developed a robust diversification platform for further functionalizations! pubs.acs.org/doi/10.1021/ja…


Standardized Approach for Diversification of Complex Small Molecules via Aryl Thianthrenium Salts | Journal of the American Chemical Society Ritter Lab Total Synthesis and Methodology Highlights pubs.acs.org/doi/10.1021/ja…

Fresh off the press at J. Am. Chem. Soc. ! 🚨 We present the next chapter in our development of N-protonated acridinium photocatalysts! A powerful yet stable photo-oxidant, our catalyst can reach un-conjugated olefins with oxidation potentials of up to ~2.35 V! pubs.acs.org/doi/10.1021/ja…


N-Protonated Acridinium Catalyst Enables Anti-Markovnikov Hydration of Unconjugated Tri- and Disubstituted Olefins | Journal of the American Chemical Society Ritter Lab Total Synthesis and Methodology Highlights pubs.acs.org/doi/10.1021/ja…

🚨Calling medicinal chemists🚨: A Suzuki het-het coupling sometimes does not require complex ligands. An air stable Ni would do. Amazing work from Rakan Saeb Rakan Saeb and Byeongdo Roh. onlinelibrary.wiley.com/doi/10.1002/an…
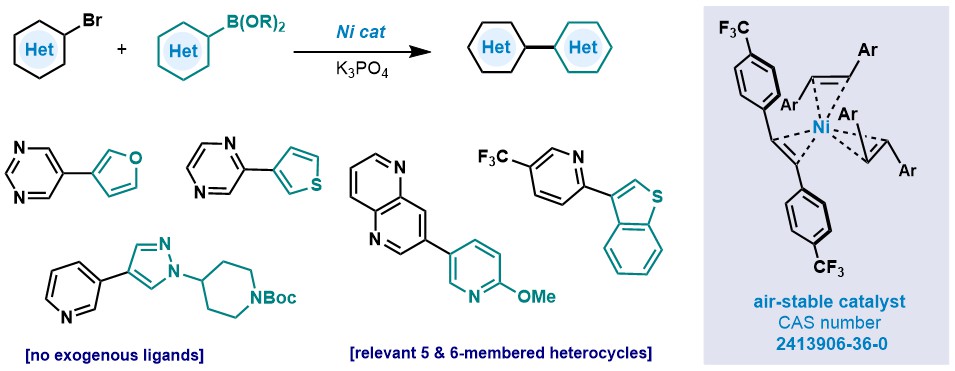



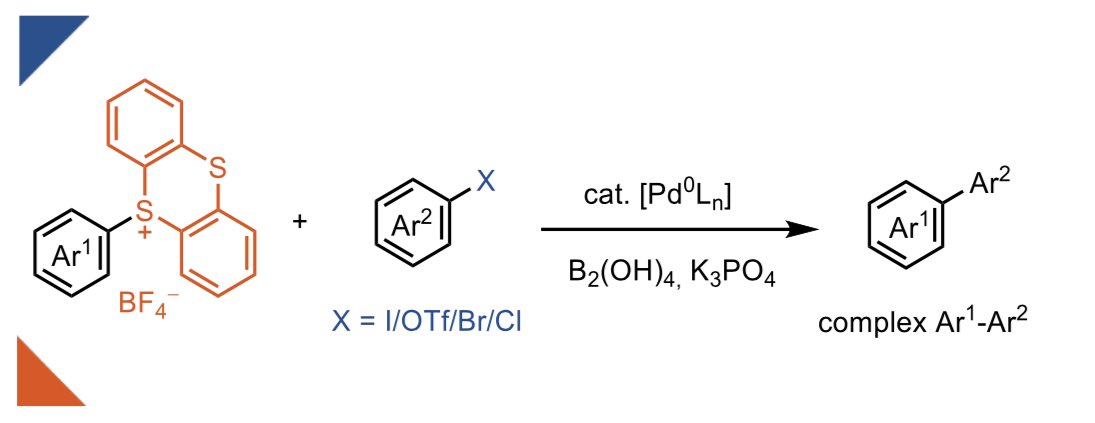
In our new publication in Angewandte Chemie, we demonstrate how the ultra-fast rate of oxidative addition of aryl-TTs to Pd(0) can be used to achieve cross-electrophile coupling with aryl-I/Br/OTf/Cl! Open access 🔓! onlinelibrary.wiley.com/doi/10.1002/an…

Nitrate reduction meets palladium – in our new Angewandte Chemie paper, we show how the generation of aryl diazonium salts as fleeting intermediates via nitrate reduction can be coupled with Pd-catalysis in a Suzuki-type cross coupling reaction! onlinelibrary.wiley.com/doi/10.1002/an…


The nitrate reduction saga continues... Very happy that the 2nd follow-up paper of our nitrate reduction strategy is finally out in J. Am. Chem. Soc. ! 🥳 open access 🔓 pubs.acs.org/doi/10.1021/ja…

Nitrate reduction at 25 °C? A little iron is all it takes! 🪄 The use of cheap iron(III) nitrate (30€ per kg!) enables the in-situ generation of aryldiazonium salts which can be engaged in sulfonylation & fluorination reactions! Out now in J. Am. Chem. Soc. 🔓 pubs.acs.org/doi/10.1021/ja…
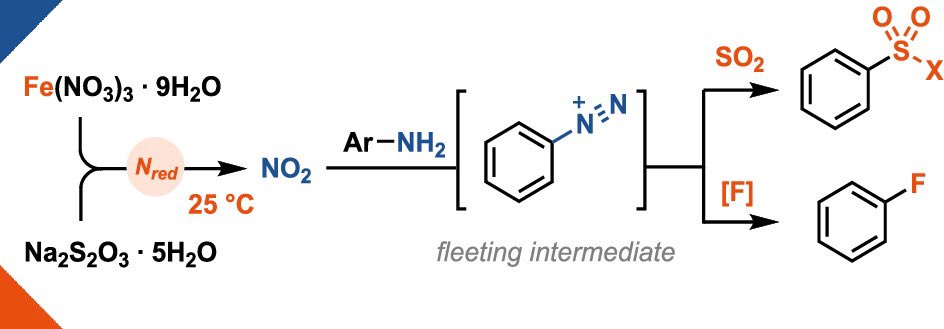

Iron-Mediated Nitrate Reduction at Ambient Temperature for Deaminative Sulfonylation and Fluorination of Anilines | Journal of the American Chemical Society Ritter Lab Total Synthesis and Methodology Highlights pubs.acs.org/doi/10.1021/ja…

New Angewandte Chemie article alert! 👀 We expand the chemical space accessible from BCP-TT reagents via a reductive cross-coupling reaction with alkyl bromides using copper/photoredox catalysis! Open access 🔓 onlinelibrary.wiley.com/doi/10.1002/an…


Kharasch addition but with a little twist 🪄 In our new J. Am. Chem. Soc. paper, the use of a phosphine-ligated Cu-complex enables the use of unstabilized, nucleophilic alkyl radicals and electron-deficient alkenes for a Kharasch-type haloalkylation reaction! 🔓 pubs.acs.org/doi/10.1021/ja…


After a multi-year journey to bring thianthrenation-like chemistry to water, we now present a selenoxide reagent that can be used to perform single atom modifications selectively on tyrosine residues in proteins or peptides! Out now in Nature Chemistry! nature.com/articles/s4155…


Now online & open access: Article by Zibo Bai, Zikuan Wang, Thomas Hin-Fung Wong & Tobias Ritter Ritter Lab Thianthrenium-enabled modular synthesis of bicyclo[1.1.1]pentanes nature.com/articles/s4416…

Nucleophilic substitution at a neopentyl-like electrophile ⁉️ Our new, bench-stable, bifunctional Iodo-BCP-TT reagent makes it possible! Our new, highly modular platform to access a broad range of BCP isosteres is now out in Nature Synthesis! ⬇️⬇️⬇️ nature.com/articles/s4416…



Selenoxide 🤝 DNA-encoded libraries! 🧬 Out now in Nature Chemistry, we present a selenium linchpin that can be introduced site-selectively into DNA conjugates and subsequently functionalized! Open access! 🔓 nature.com/articles/s4155…


