IMI_SISAQOL
@sisaqol
Setting International Standards in Analysing Patient Reported Outcomes and Quality of Life Endpoints in Cancer Clinical Trials
ID: 1356990932936228865
https://www.sisaqol-imi.org/ 03-02-2021 15:41:21
130 Tweet
256 Followers
114 Following


Excited for a full day of new #SISAQOL recommendations for the statistical analysis of #patientreportedoutcomes in cancer clinical trials! IMI_SISAQOL














The fearless leaders of #SISAQOL: Anders Ingelgaard & Madeline Pe — thank you for your vision and work to enhance PRO statistical analysis for everyone! IMI_SISAQOL





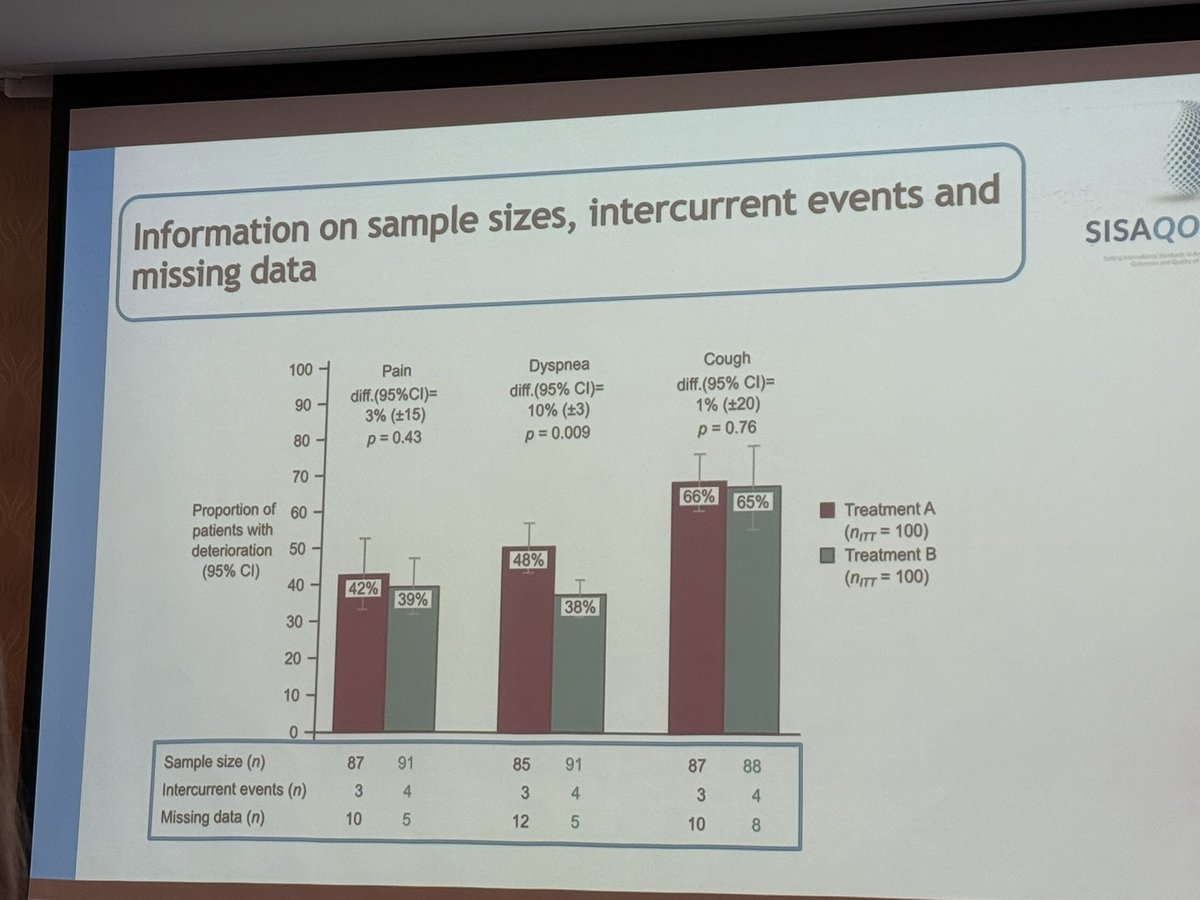
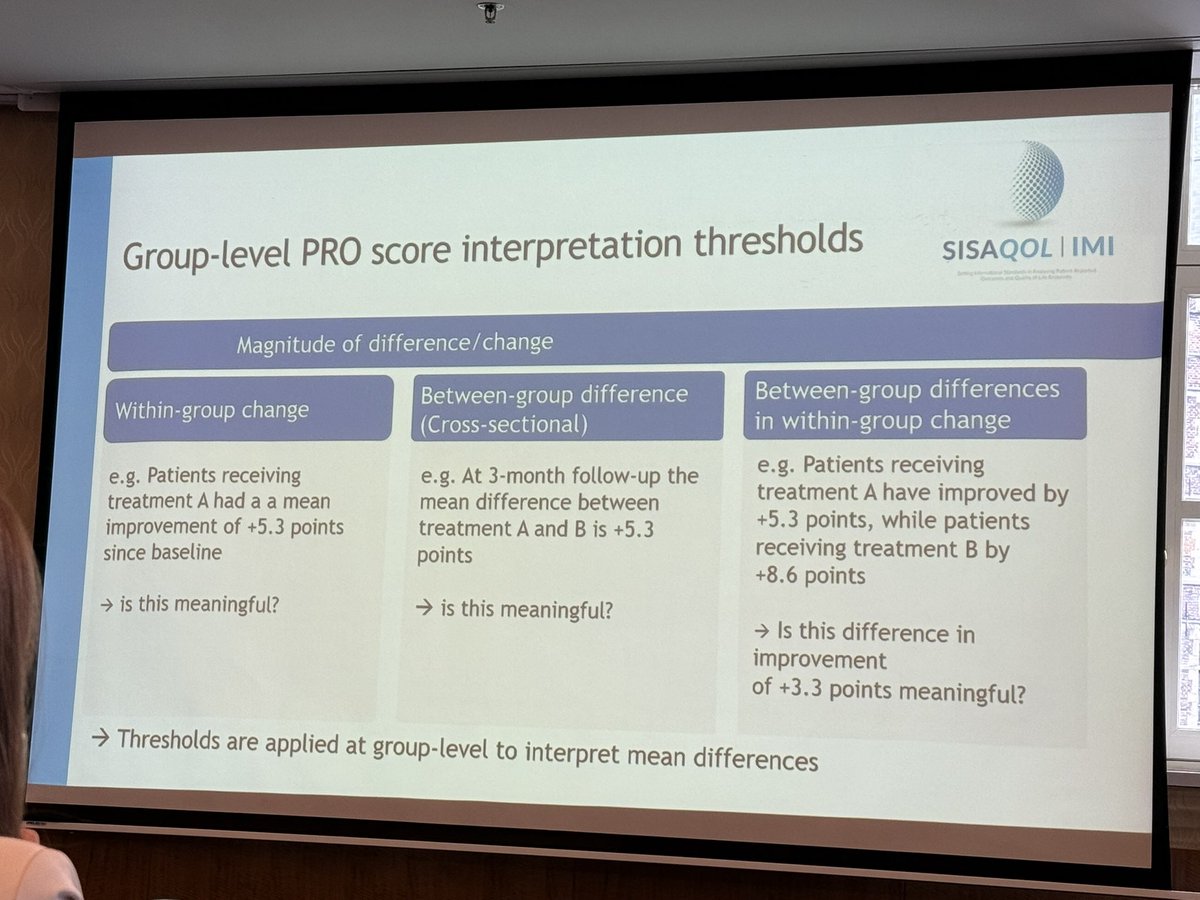
🚨 New Publication Alert! 🚨 Our latest publication in BMC Medical Research Methodology explores handling missing patient-reported outcome data in the presence of intercurrent events. Aligning analysis with the estimand framework is key! 📊 Read more: bmcmedresmethodol.biomedcentral.com/articles/10.11…

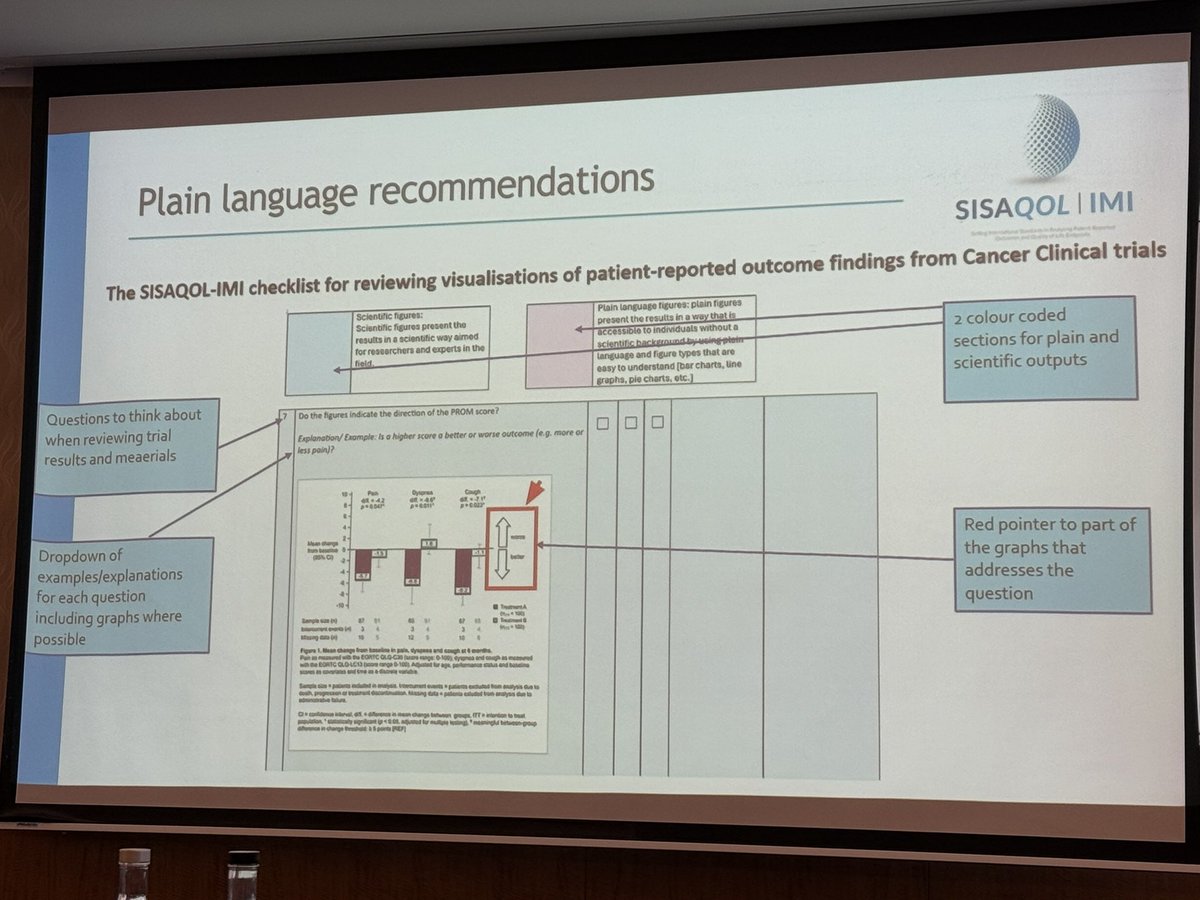
We’re thrilled to announce that our consortium will be presenting the SISAQOL-IMI recommendations at the Royal Statistical Society Conference 2025 in Edinburgh this September! We can't wait to share our insights and engage in thought-provoking discussions. Stay tuned for more details 📊📢