Nikos Kaplaneris
@synthnick
Postdoctoral researcher with Prof. Noël @NoelGroupUvA 🇳🇱 | PhD with Prof. Ackermann @aztul 🇩🇪
ID: 355536474
https://scholar.google.com/citations?user=3Do25boAAAAJ&hl 15-08-2011 14:23:01
499 Tweet
497 Followers
751 Following





Excited to share this new primer on Flow Chemistry! Learn the foundations of flow synthesis in collaboration with Duncan Browne Anna Slater (she/her) and Baumann Research Group Nature Reviews Methods Primers nature.com/articles/s4358…


Cracking one of synthesis’s toughest nuts: dioxygen activation! In collaboration with the Costas Lab Miquel Costas , our DKP-Mn combo hits selective C–H oxidation of alkanes with sharp chemoselectivity under aerobic conditions. Many congrats Kiki! pubs.acs.org/doi/10.1021/ja…

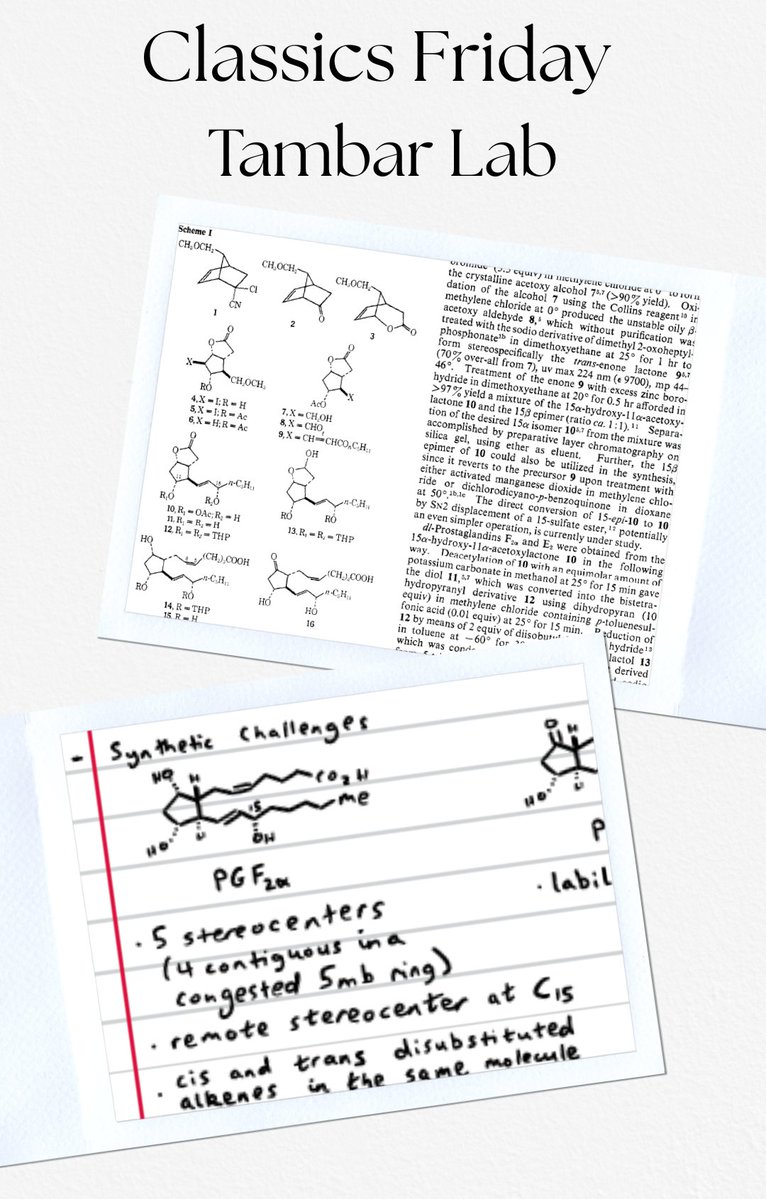
Out today in J. Am. Chem. Soc. is the first synthesis from our group!! We report a convergent and stereoselective total synthesis of the nominal structure of Talaromyolide D. Huge congrats to Bo and Alex! NYU Chemistry Total Synthesis pubs.acs.org/doi/10.1021/ja…

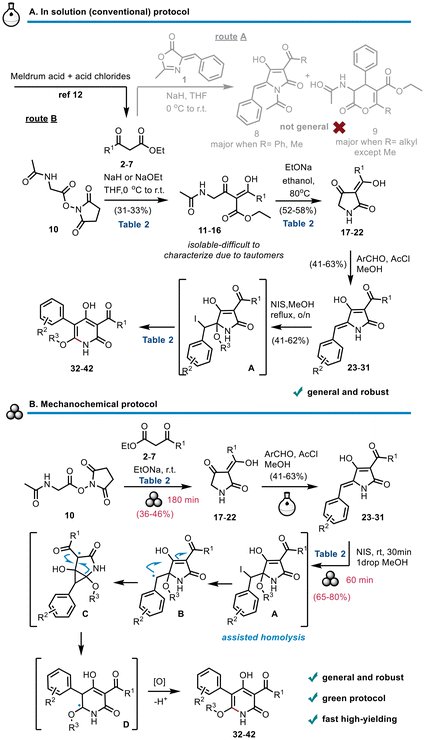
Our latest in J. Am. Chem. Soc., we report a general strategy to access antimicrobial sactipeptides by Markovnikov hydrothiolation of dehydroamino acids. Congratulations to Yiwei, Shuvendu, Steven and Yannik for their hard work! pubs.acs.org/doi/10.1021/ja…




Glad to share our recent #JOrgChem article on catalytic asymmetric Total Synthesis of naturally occurring Amaryllidaceae alkaloids, (−)-elwesine and (−)-epi-crinine! Congratulations to Satyajit Majumder Debabrata Mondal Abhishek Mondal Souvik Pal👏 pubs.acs.org/doi/10.1021/ac…



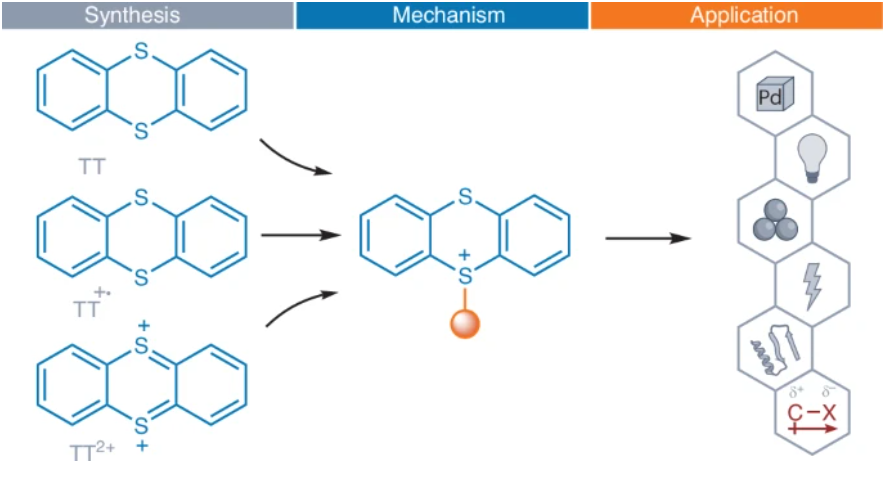
In Nature Synthesis, we provide our perspective on the blossoming of organo-TT chemistry since 2019. From fundamentals to applications, we explain how the thianthrenium substituent enables chemistry that in part goes beyond that of other (pseudo)halides. rdcu.be/eIEM6

Excited to share that we have received the Hansen Family Award: Groundbreaking Research: Chemist Lutz Ackermann Receives Bayer Foundation’s Hansen Family Award | Bayer Foundation Uni Göttingen bayer-foundation.com/groundbreaking…

📢 We are recruiting PhD candidates to join our dynamic research group at Uni of Nottingham #WeAreUoN ! ⚗️🧪🧑🔬👩🔬💡 Check out our website silviresearch.com for more info and to find out how to apply! 👀

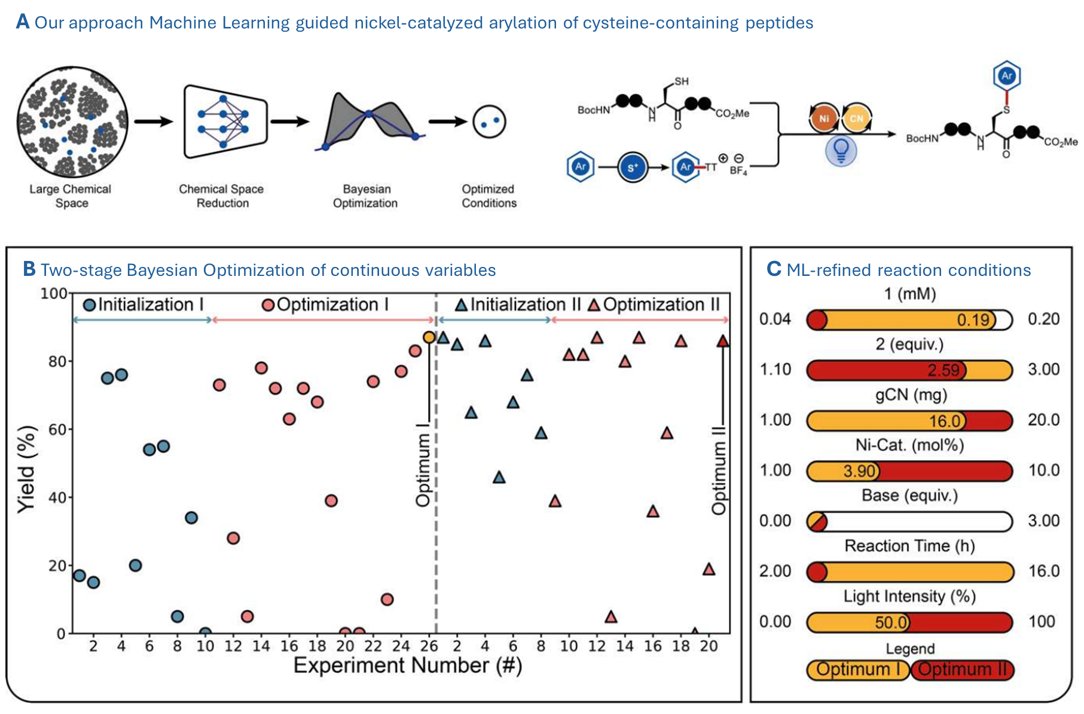
We just achieved the first site-selective cysteine functionalization with EBX reagents! J. Am. Chem. Soc. pubs.acs.org/doi/10.1021/ja…

⚡️We are happy to share our recent work in Angewandte Chemie with a collaboration with Kim group at CNU. By adjusting electrochemical conditions, we access haliranium-mediated C–O bond or amidyl-radical-driven C–N bond formation, supported by CV, kinetic, and computational studies.