
Thomas Hwang, MD
@thomasmd
new medicines, digital health, and uro oncology @bwhurology @danafarber
ID: 1361285146645790722
15-02-2021 12:04:34
36 Tweet
164 Followers
46 Following

Thank you Equitable Growth for including our project (w/ Josh Feng & Thomas Hwang, MD) in this year’s funding round! It’s a privilege to be part of this great group of researchers

New study: No of drug indications granted accelerated approval increased in US&Europe, only approx 1/3 with high value. Universität Zürich, Program On Regulation, Therapeutics, And Law, Brigham and Women's Hospital Department of Urology, Thomas Hwang, MD jamanetwork.com/journals/jama-… Funded by Swiss National Science Foundation, Krebsforschung Schweiz Swiss national news: srf.ch/news/schweiz/w…

The Swiss National Science Foundation Swiss National Science Foundation is honoured to help choose the winners of the Swiss Science Prizes. And the 2022 winners are .... Ursula Keller of ETH Zurich and Kerstin Noëlle Vokinger of University of Zurich. Wonderful, congratulations! #MarcelBenoist #Latsis


Thank you again Toni Choueiri, MD Dana-Farber Lank Center for Genitourinary Oncology for having me today for an excellent discussion and Adam Kibel for the v kind introduction.


Thank you Celine Castronuovo for talking with me in Bloomberg on reforms to accelerated approval; earlier in Medscape Endpoints News (more in recent work The Lancet JAMA Health Forum) news.bloomberglaw.com/health-law-and…


New paper Thomas Hwang, MD in New England Journal of Medicine proposing federal incentives for equity in clinical trials of new medicines, building on recent U.S. FDA guidance and landmark National Academies report 🔗: nejm.org/doi/full/10.10… with Otis Brawley in NEJM


A MUST read this weekend with our Brigham and Women's Hospital Department of Urology Super Star intern Thomas Hwang, MD & globally-recognized expert Otis Brawley in NEJM => federal incentives for equity in clinical trials of new drugs, building on recent U.S. FDA guidance & National Academies report 🔗: nejm.org/doi/full/10.10…


Important @NEJMPerspec from Thomas Hwang, MD at Program On Regulation, Therapeutics, And Law on improving quality and generalizability of clinical trials!: New Federal Incentives for Diversity in Clinical Trials | NEJM nejm.org/doi/full/10.10…

Thanks Nicole DeFeudis for talking with me about our NEJM piece and proposed framework to ensure more representative clinical trials. in Endpoints News endpts.com/are-incentives…



New paper by our resident, Thomas Hwang, MD, in JAMA proposing a framework for World Health Organization (WHO) to improve global access to new medicines, particularly new cancer therapies, in light of evolving innovation landscape. 🔗:jamanetwork.com/journals/jama/… with Kerstin Noëlle Vokinger Aaron Kesselheim


#Viewpoint proposes restructuring World Health Organization (WHO)'s Model List of Essential Medicines to formally remove consideration of cost and cost-effectiveness from the expert committee reviews of clinical effectiveness, safety, and public health value. ja.ma/3syL1AE


Congratulations to Quoc-Dien Trinh, MD, MBA, who has been named Section Chief of Urology at Brigham and Women's Faulkner Hospital effective Tuesday, November 1st! 🔗:brighamandwomensfaulkner.org/about-bwfh/new…


The FDA will soon require companies and researchers to detail how they plan to enroll a diverse clinical-trial participant pool before they begin late-stage trials. Read more in my latest for nature, with Jennifer E. Miller, Marian Knight MBE, Thomas Hwang, MD: nature.com/articles/d4158…

Thank you nature Max Kozlov 🇺🇦 for covering our NEJM piece and the new law on diversity in clinical trials. Looking forward to presenting at Multi-Disciplinary conference Dana-Farber Lank Center for Genitourinary Oncology and discussing implications for trialists. nature.com/articles/d4158…


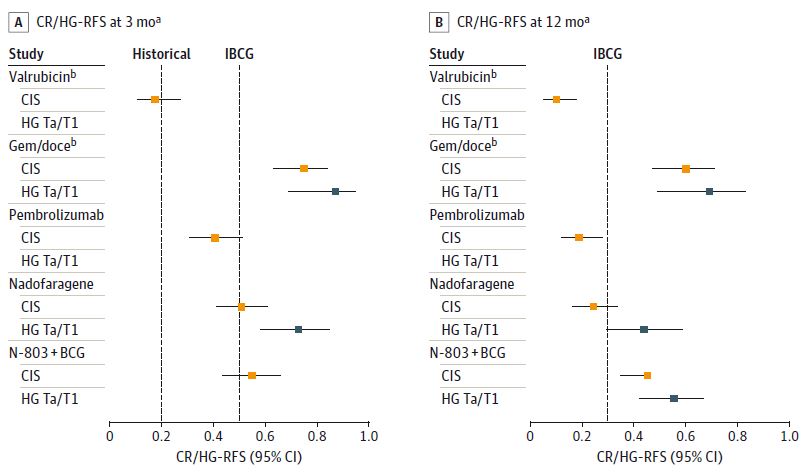
new piece JAMA Oncology on new therapies for BCG-unresponsive NMIBC and modernizing FDA guidance on trial design. Mark Preston Benjamin J. Davies MD jamanetwork.com/journals/jamao…

Congrats to Thomas Hwang, MD (Thomas Hwang, MD) of Dana-Farber and Brigham and Women's Hospital Department of Urology on being recognized as a 2023 #StatWunderkind STAT Dana-Farber Lank Center for Genitourinary Oncology Toni Choueiri, MD ms.spr.ly/60129vhHq

So proud of BWH urology resident Tom’s work. So important to have smart clinicians focused on policy. Brigham and Women's Hospital Department of Urology Thomas Hwang, MD statnews.com/wunderkinds-20…


